Swelling and Characterization Behavior of Anti-salt Superabsorbent based on Carboxymethyl Cellulose-g-PAA-co-BuMC Hydrogel
Mohammad Sadeghi¹ and Mojgan Yarahmadi²
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak (Iran). ²Department of English, Humaniti Faculty, Islamic Azad University, Arak Branch, Arak (Iran).
A novel superabsorbent hydrogel was synthesized via crosslinking graft copolymerization of acrylacid (AA) abd buthylmethacrylate (BuMC) onto Carboxymethyl cellulose (CMC) backbones in a homogeneous solution. The methylenebisacrylamide (MBA) and Ceric amounium sulfate (CAN) were used as water-soluble crosslinker and initiator, respectively. Evidence of grafting was obtained by comparing FTIR,SEM spectra of CMC and the graft copolymer as well as solubility characteristics of the products. A mechanism for the superabsorbent hydrogel formation was also suggested. The affecting parameters onto swelling capacity of the synthesized hydrogel, i.e., concentration of AA/BuMC waight ratio, MBA as well as reaction temperature were systematically optimized for obtaining maximum absorbency as possible as. The swelling capacity of hydrogels was also measured in various salt solutions (LiCl, NaCl, KCl, MgCl2, CaCl2 , SrCl2, BaCl2, and AlCl3). Due to high swelling ability in salt solutions, the hydrogel may be referred as “anti-salt superabsorbent” polymers. The overall activation energy for the graft copolymerization reaction was found to be 374 kJ/mol. The swelling kinetics of the hydrogels in distilled water was preliminary investigated.
KEYWORDS:Superabsorbetn; Carboxymethyl cellulose; Hydrogel
Download this article as:| Copy the following to cite this article: Sadeghi M, Yarahmadi M. Swelling and Characterization Behavior of Anti-salt Superabsorbent based on Carboxymethyl Cellulose-g-PAA-co-BuMC Hydrogel. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Sadeghi M, Yarahmadi M. Swelling and Characterization Behavior of Anti-salt Superabsorbent based on Carboxymethyl Cellulose-g-PAA-co-BuMC Hydrogel. Available from: http://www.orientjchem.org/?p=11688 |
Introduction
In recent years, increasing interest in natural-based superabsorbent hydrogel has developed mainly due to high hydrophilicity, biocompatibility, non-toxicity, and biodegradability of biopolymers. These materials are defined as crosslinked macromolecular networks that can absorb water or physical fluids up to many times of their own weight in a short time, but are not dissolved when brought into contact with water(1). The absorbed fluids are hardly removable even under some pressure. Because of excellent characteristics, superabsorbent hydrogels are widely used in many fields, such as agricultural and horticultural, disposable diapers, feminine napkins, pharmaceuticals and medical applications(2-4). This accounts for increase in the worldwide production of superabsorbent polymers (SAPs) from 6000 tons in 1983 to 450000 tons in 1996(2). Nowadays, the worldwide production of SAPs is more than one million tons in year. Hence, synthesis and investigation of specific and new superabsorbent hydrogels with high absorbency, mechanical strength and initial absorption rate, has been the goal of several research groups in the past decades(5-8).
Because of their exceptional properties, i.e. biocompatibility, biodegradability, renewability, and non-toxicity, polysaccharides are the main part of the natural-based superabsorbent hydrogels. Carboxymethylcellulose (CMC), an anionic water-soluble polysaccharide, is important modified cellulose which is used in various fields such as detergent, food, paper and textile industries. The application potential of CMC has been demonstrated because of its promising characteristics including those of mentioned above.
Most of ionic hydrogels sometimes undergo a volume phase in response to a little change in surrounding conditions such as heat, pH, electric field, chemical environments, etc. These hydrogels response to external stimuli are often referred to as “intelligent” or “smart” hydrogels. They have important applications in the field of medicine, pharmacy, and biotechnology. Among these, pH-sensitive hydrogels have been extensively investigated for potential use in site-specific delivery of drugs to specific regions of the gastrointestinal tract and have been prepared for delivery of low molecular weight protein drugs(9-10).
Free radical graft copolymerization of vinylic monomers onto polysaccharide backbones followed by crosslinking of their chains is a well-known method for synthesis of these polysaccharide-based networks. The first industrial superabsorbent hydrogel, hydrolyzed starch-g-polyacrylonitrile (HSPAN), was synthesized using this method via ceric-induced graft copolymerization of acrylonitrile onto starch followed by crosslinking alkaline hydrolysis of the nitrile groups of the produced graft copolymer(11). Radical polymerization, however, has several disadvantages. Reproducibility of this method is poor, and there is little control over the grafting process, so that the molecular weight distribution is polydisperse. In addition, the necessity for inert gases, e.g. argon, for preparing of oxygen-free atmosphere and use of toxic and expensive crosslinker agents are another disadvantages of free radical polymerization reactions. These problems have been reviewed in detail(12).
To the best of our knowledge based on a precise survey of the Chemical Abstracts, there is no published report on the synthesis of a superabsorbing hydrogel via simultaneously graft copolymerization acrylic acid and buthylmetacrylate onto CMC. Hence,the present work reveals graft copolymerization of acrylic acid and buthylmetacrylate monomers onto CMC in the presence of methylenebisacrylamide (MBA) as a crosslinking agent. The reaction variables affected the swelling capacity of CMC-g-PAA-co-BuMC Superabsorbent hydrogels was studied..
Experimental
Materials
The polysaccharide, Carboxymethylcellulose (CMC, degree of substitution 0.52, from Condinson Co., Denmark) was of analytical grade and was used as received. Acrylic acid (AA, Merck), Buthylmethacrylate (BuMC, Merck) were used after vacuum distillation. Ceric amounium sulfate (CMC, Merck) was used without purification. Methylenebisacrylamide (MBA, Fluka), was used as recieved. All other chemicals were of analytical grade. Double distilled water was used for the hydrogel preparation and swelling measurements.
Preparation of hydrogel
A weighed amount of CMC(1.0 g) was dissolved in 50 mL of distilled water in a 100 mL two-necked flask equipped with magnetic stirrer, immersed into a thermostated water bath, preset at 65oC. An inert gas (argon) was gently bubbled into the reactor to remove the oxygen during the graft copolymerization reaction. After 15 min, various amounts of BuMC and AA monomers simultaneously were added to the reaction mixture at once. MBA as a crosslinker was added to the reaction mixture after the addition of monomers and the mixture was continuously stirred for for 10 min under argon. then a given volume of freshly prepared solution of CAN(0.050 g in 2 mL water) was added and the graft copolymerization reaction was conducted for 2 h. Finally, the resulted product was precipated by pouring the reaction mixture solution into 250 mL of methanol, and the precipitate was filtered and repeatedly washed with methanol. After complete dewatering for 24 h, the product dried at 50 oC.
Infrared Analysis
The samples were crushed with KBr to make pellets. Spectra were taken on an ABB Bomem MB-100 FTIR spectrophotometer.
Swelling Measurements
A CMC-g-PAA-co-PBuMC sample (0.10 g) was put into a weighed teabag and immersed in 100 mL distilled water and allowed to soak for 2 h at room temperature. The equilibrated swollen gel was allowed to drain by removing the teabag from water and hanging until no drop drained (~30 min.). The bag was then weighed to determine the weight of the swollen gel. The absorbency (equilibrium swelling) was calculated using the following equation:
Absorbency = (Ws – Wd)/Wd (1)
where Ws and Wd are the weights of the swollen gel and the dry sample, respectively. So, absorbency was calculated as grams of water per gram of resin (g/g). The accuracy of the measurements was ±2%.
Swelling in Salt Solutions
Absorbency of the CMC-g-PAA-co-PBuMC hydrogel sample was evaluated in 0.15 molar solutions of (LiCl, NaCl, KCl, MgCl2, CaCl2, SrCl2, BaCl2, and AlCl3) according to the above method described for swelling measurement in distilled water.
Swelling Kinetics
Hydrogel samples (40-60 mesh, 0.10 g) were poured into numbers of weighed teabags and immersed in 100 mL distilled water. At consecutive time intervals, the water absorbency of the samples was measured according to the equation (1).
Results and Discussion
Synthesis and Characterization
PAA and PBuMC monomers was simultaneously grafted onto CMC in a homogenous medium using CMC as a radical initiator and MBA as a crosslinking agent under an inert atmosphere.
The mechanism of co polymerization of AA and BuMC onto CMC in the presence of MBA is shown in Scheme 1. In the first step, the thermally dissociating initiator, i.e. CAN, is decomposed under heating (65oC) and Then pair redox ce3+-ce4+ disconnect C-C bond from the CMC backbones to form corresponding macroinitiators. These macroradicals initiate grafting of AA and BuMC onto CMC backbones leading to a graft copolymer. Since a crosslinking agent, e.g. MBA, is presented in the system, the copolymer comprises a crosslinked structure. The superabsorbency of this hydrogel in distilled water and various saline solutions were investigated.
For identification of the hydrogel, infrared spectroscopy was used. Figure 1 shows the IR spectroscopy of CMC-g-PAA-co-PBuMC hydrogel. The superabsorbent hydrogel product comprises a CMC backbone with side chains that carry carboxamide functional groups that are evidenced by peaks at 1636 cm-1. In fact, In the spectrum of the hydrogel (Fig. 1-b), new peak is appeared at 1716 cm-1 that may be attributed to carbonyl esther bending related to Buthylmethacrylate monomer.
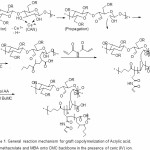 |
Scheme 1: General reaction mechanism for graft copolymerization of Acrylic acid, Buthylmethacrylate and MBA onto CMC backbone in the presence of ceric (IV) ion. Click here to View scheme |
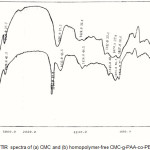 |
Figure 1: FTIR spectra of (a) CMC and (b) homopolymer-free CMC-g-PAA-co-PBuMC. |
 |
Figure 2: SEM photograph of the optimized superabsorbent hydrogel. Surface of pure carboxymethylcelloluse(a); Surface of porous hydrogel(b). |
Optimization of effecting parameters onto swelling capacity
Effect of crosslinker concentration on the swelling capacity
Crosslinks have to be present in a hydrogel in order to prevent dissolution of the hydrophilic polymer chains in an aqueous environment. Efficiency of crosslinker incorporation controls the overall crosslink density in the final hydrogel. The effect of the extent of crosslinking on water absorbency of CMC-g-PAA-co-PBuMC hydrogel is shown in Figure 3. In this series reaction. the results indicate power law behaviors of swelling-[MBA], so that the lower the crosslinking agent, the decreased water absorbency will be. Higher crosslinker concentration produce more crosslinked points in polymeric chains and increases the extent of crosslinking of the polymer network, which results in less swelling when it is brought into contact with solvent. Similar observations have been reported in the literature(6, 14). The swelling capacity for hydrogel is 331 g /g, at a fixed MBA concentration of 0.1 mol/L.
Effect of AA/BuMC Weight Ratio on the swelling capacity
The substrate CMC was treated with different amounts of AA/BuMC ratio (0.55-5.5 mol/L), while the rest of variables was unchanged. The maximum water absorbency (367.0 g/g) was obtained at AA/BuMC= 3.0 (Fig.4). The initial increase in water absorbency could be originated from the greater availability of monomer molecules in the vicinity of the chain propagating sites of CMC macroradicals. In addition, higher AA content enhances the hydrophilicity of the hydrogel that, in turn, causes a stronger affinity for more absorption of water. Indeed in high concentration of charged ionic groups existing in copolymer chains along with increase of NaAA in the hydrogel increases the swelling capacity due to osmosis and charge repulsion. In other words, the presence of more ionic groups in the polymer chains results in increased swelling, because the ionic groups are more strongly solvated than non-ionic groups in the aqueous medium. Similar conclusions were reported by other investigators. A further increase of monomer concentration, however, results in decreased absorbency.It is probably due to (a) preferential homopolymerization over graft copolymerization, (b) increase in viscosity of the medium which hinders the movement of free radicals and monomer molecules, (c) the enhanced chance of chain transfer to monomer molecules, and (d) non grafted poly(AA-BuMC) chains. The latter reason is in close agreement with similar starch-based superabsorbents reported by Athawale et al (17, 18).
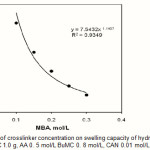 |
Figure 3: Effect of crosslinker concentration on swelling capacity of hydrogel.Reaction conditions: CMC 1.0 g, AA 0. 5 mol/L BuMC 0. 8 mol/L, CAN 0.01 mol/L, 65 °C, 120min Click here to View figure |
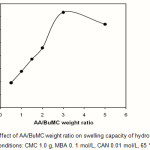 |
Figure 4: Effect of AA/BuMC weight ratio on swelling capacity of hydrogel.
Reaction conditions: CMC 1.0 g, MBA 0. 1 mol/L, CAN 0.01 mol/L, 65 °C, 120 min. |
Effect of bath temperature on swelling capacity
To examine of bath temperature on swelling capacity of hydrogel, the process was carried out from 30 to 75 oC. as shown in Figure 5, swelling capacity of hydrogel shows an increase with temperature, reaching a maximum at 60 oC. This behavior can be attributed to:
Better decomposition of the initiator, giving rise to more free radicals.
Increasing rate of graft copolymerization of hydrophilic AcA and BuMC monomers onto CMC(19-22).
A further increase in temperature could result in the enhanced mobility of macroradicals, which may lead to termination, hence the grafting of AcA and BuMC onto CMC decreases and the swelling capacity showed a decrease above the optimum temperature.
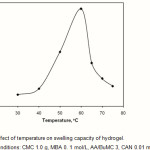 |
Figure 5: Effect of temperature on swelling capacity of hydrogel.
Reaction conditions: CMC 1.0 g, MBA 0. 1 mol/L, AA/BuMC 3, CAN 0.01 mol/L, 65 °C. |
Swelling in salt solutions
The water absorbency for the present CMC-g-PAA-co-BuMC hydrogels in NaCl, CaCl2 and AlCl3 solutions with different concentration are shown in Figure 6. CMC-g-PAA-co-BuMC hydrogel comprises carboxylate groups in the CMC backbones. So the resulted hydrogels are polyelectrolyte superabsorbent(23).
As shown in this Figure, the water absorbency of hydrogel in salt solutions is lower than that of distilled water. This well-known phenomenon, commonly observed in the swelling of ionic hydrogels, is often attributed to a charge screening effect of the additional cations causing a non-perfect anion-anion electrostatic repulsion, led to a decreased osmotic pressure (ionic pressure) difference between the hydrogel network and the external solution.
As shown in this figure, the tendency of water absorbency for the hydrogel is in the order of Na+> Ca2+ > Al3+ cations in salt solutions with same concentration and same Cl– counter ions of cations. It may be explained by complexing ability arising from the coordination of the multivalent cation with carboxylate groups of CMC-g-PAA-co-BuMC (Scheme 1), so the crosslinking density increases in the hydrogel and swelling capacity decreases.
To achieve a comparative measure of sensitivity of the hydrogels to the kind of aqueous fluid, we defined a dimensionless swelling factor, f, as follows:
f= 1-(Absorption in a given fluid/Absorption in deionized water) (2)
The effect of increasing cation charge on ultimate absorption for the different CMC-g-PAA-co-BuMC superabsorbents can be found from the values, so that the lower the cation charge, the lower the salt sensitivity. Salt coefficient factors for these hydrogels are low. In the response to these phenomena, it can be mentioned that in ionic hydrogel, the swelling capacity in salt solution is low because of high charge screening effect and complexing with cations. In CMC-g-PAA-co-BuMC hydrogel, there was low ionic group (carboxylate groups on the CMC backbones) in comparisons to the synthetic polyacrylate hydrogels and screening effect cannot cause to low swelling capacity. The swelling of hydrogel affected by the presence of amide groups (non-ionic), and so the salt coefficient is low(24-26).
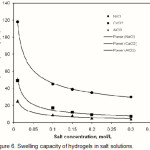 |
Figure 6: Swelling capacity of hydrogels in salt solutions. |
Swelling kinetic
The water absorbency of the CMC-g-PAA-co-BuMC hydrogel was measured against time at distilled water at 25 oC and is shown in Figure 7. Initially, the rate of the water uptake sharply increases and then begins to level off. It is indicated that the water absorbency of the hydrogel reaches 140 H2O /g within 2 min, and 174 g H2O g/g within 60 min.
According to Figure 8,the swelling values versus swelling time follows a power low trend. A ˝ Voigt-based model ˝ (Fig. 1) may be used for fitting the data [23].
![]()
St is the swelling in time t, Se is the equilibrium swelling (power parameter) and τ is the rate parameter.The power parameter according to Figure 7 is 174 g/g. For calculate the rate parameter, by using the above formula and a little rearrangement, one can be plot versus time (t). The slope of the straight line fitted (slope= -1/ τ) gives the rate parameter (τ 0.9 min). It should be pointed out that the rate parameter (which is a measure of the swelling rate) is totally dependent upon the particle size of the swelling gel . The average size of the hydrogel particles used in this experiment was 250-350 μm(26).
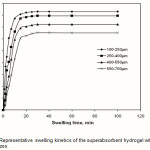 |
Figure 7: Representative swelling kinetics of the superabsorbent hydrogel with various particle sizes. |
Conclusion
The grafting of simultaneously acrylic acid and buthylmetacrylate onto CMC was carried out using CAN as an efficient initiator in the presence of methylenebisacrylamide (MBA) as a crosslinking agent. In order to prove that AA and BuMC molecules were grafted, solubility test, FTIR and SEM spectroscopies, were used. Effect of various reaction parameters on the swelling capacity, such as the AA/BuMC waight ratio and MBA concentration as well as temperature was studied.
References
- Buchholz, F. L.; Graham, A. T. Modern Superabsorbent Polymer Technology; Elsevier: Amsterdam, (1997).
- Peppas, L. B.; Harland, R. S. In Absorbent Polymer Technology; Elsevier: Amsterdam, (1990).
- Po, R. J Macromol Sci Rev Macromol Chem Phys, C34, 607(1994).
- Hoffman, A. S. In Polymeric Materials Encyclopedia; Salamone, J. C., Ed.; CRC Press: Boca Raton, FL,; Vol. 5, p 3282(1996).
- Yazdani-Pedram, M.; Retuert, J.; Quijada, R. Macromol Chem Phys, 201, 923(2000).
- Sugahara, Y.; Takahisa, O. J Appl Polym Sci, 82, 1437(2001).
- Patel, G. M.; Trivedi, H. C. Eur Polym J, 35, 201(1999).
- Silong, S.; Rahman, L. J Appl Polym Sci, 76, 516(2000).
- Kost, J. In Encyclopedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; Wiley: New York, Vol. 1, p 445(1999).
- Peppas, N. A.; Mikes, A. G. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, Vol. 1(1986).
- U.S. Department of Agriculture. U.S. Pat. 3,981,100 (1961).
- Stannett, V. T. ACS Symp Ser, 1, 187(1982).
- Fanta, G. F.; Burr, R. C.; Doane, M. W. ACS Symp Ser, 187, 195(1982).
- Fanta, G. F.; Doane, M. W. U.S. Pat. 4,116,899 (1978).
- Yamaguchi, M.; Watamoto, H.; Sakamoto, M. Carbohydr Polym, 9, 15(1988).
- Rodehed, C.; Ranby, B. J Appl Polym Sci, 32, 3323(1986).
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Weaver, M. O.; Gugliemeli, L. A.; Doane, W. M.; Russel, C. R. J Appl Polym Sci, 15, 3015(1971).
- Silverstein, R. M.; Webster, F. X. Spectrometric Identification of Organic Compounds, 6th ed.; Wiley: New York, (1998).
- Sjostrom, E. Fundamental of Carbohydrate Chemistry and Wood Chemistry: Fundamentals and Applications; Academic: San Diego, (1981).
- Flory, P. J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, (1953).
- Castel, D.; Ricard, A.; Audebert, R. J Appl Polym Sci, 39, 11(1990).
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Hermans, J. J.; In Flow Properties of Disperse Systems, Wiley Interscience, New York, (1953).
- Lee, W. F.; Yuan, W. Y. J Appl Polym Sci, 77, 1760(2000).
- Park, S. E.; Nho, Y. C.; Lim, Y. M.; Kim, H. J Appl Polym Sci, 91, 636(2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









