Study of Physical Chemistry on Biosorption of Nickel by Using Chlorella Pyrenoidosa
Hassan Rezaei*, Satish D. Kulkarni and Praveen G. Saptarshi
Environmental Sciences Department, University of Pune, Pune India.
In the present study, the biomass generated from the dried Chlorella pyrenoidosa was used for evaluating the biosorption characteristics of Ni ions in aqueous solutions. Batch adsorption experiments were performed on these leaves and it was found that the amount of metal ions adsorbed increased with the increase in the initial metal ion concentration. In this study effect of agitation time, initial metal ion concentration, temperature, pH & biomass dosage were studied. Maximum metal uptake was observed at pH= 5. Maximum metal uptake (qmax) was 142.86 mg/g .The biosorption followed both Langmuir and Freundlich isotherm model .The adsorption equilibrium was reached in about 1 h. The kinetic of biosorption followed the second - order rate. The biomass could be regenerated using 0.1 M HNO3. A positive value of ï„H° indicated the endothermic nature of the process. A negative value of the free energy (ï„G°) indicated the spontaneous nature of the adsorption process. A positive value of ï„S° showed increased randomness at solid-liquid interface during the adsorption of heavy metals, it also suggests some structural changes in the adsorbate and the adsorbent. FTIR Spectrums of Chlorella pyrenoidosa revealed the presence of hydroxyl, amino, carboxylic and carbonyl groups. The scanning electron micrograph (SEM) clearly revealed the surface texture and morphology of the biosorbent.
KEYWORDS:Biosorption; Chlorella pyrenoidosa; Ni; Isotherm models; Kinetic
Download this article as:| Copy the following to cite this article: Rezaei H, Kulkarni S. D, Saptarshi P. G. Study of Physical Chemistry on Biosorption of Nickel by Using Chlorella Pyrenoidosa. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Rezaei H, Kulkarni S. D, Saptarshi P. G. Study of Physical Chemistry on Biosorption of Nickel by Using Chlorella Pyrenoidosa. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24957 |
Introduction
Discharge of heavy metals from metal processing industries is known to have adverse effects on the environment. Biosorption of heavy metals by metabolically inactive biomass of microbial organisms is an innovative and alternative technology for removal of these pollutants from aqueous solution. Presence of heavy metals in the aquatic system is posing serious problems and Nickel has been used in many industrials and removal of Ni ions from waste waters is significant. Biosorption is one of the economic methods that used for removal of heavy metals nickel is a compound that occurs in the environment only at very low levels. Humans use nickel for many different applications. The most common application of nickel is the use as an ingredient of steal and other metal products. It can be found in common metal products such as jewellery. An uptake of too large quantities of nickel has the following consequences: Higher chances of development of lung cancer, nose cancer, larynx cancer and prostate cancer, Sickness and dizziness after exposure to nickel gas, Lung embolism, Respiratory failure, Birth defects, Asthma and chronic bronchitis , Allergic reactions such as skin rashes, mainly from jewellery and Heart disorders. Electroplating is one important process involved in surface finishing and metal deposition for better life of articles and for decoration. Although several metals can be used for electroplating, nickel, copper, zinc and chromium are the most commonly used metals, the choice depending upon the specific requirement of the articles. During washing of the electroplating tanks, considerable amounts of the metal ions find their way into the effluent. Ni (II) is present in the effluents of silver refineries, electroplating, zinc base casting and storage battery industries [1]. Higher concentrations of nickel cause cancer of lungs, nose and bone. Dermatitis (Ni itch) is the most frequent effect of exposure to Ni, such as coins and jewellery. Acute poisoning of Ni (II) causes headache, dizziness, nausea and vomiting, chest pain, tightness of the chest, dry cough and shortness of breath, rapid respiration, cyanosis and extreme weakness [2,3 and 4].Recently, the commonly used methods applied to remove excessive Nickel from aqueous solutions have included ion exchange, chemical precipitation, activated carbon adsorption, evaporation and membrane processes. However, these methods were found to be either inefficient or expensive when metal ions exist in low concentrations (<100 mg/L) and may also be associated with the generation of secondary environmental problems from waste disposal[5] .Biosorption is the binding and concentration of heavy metals from aqueous solutions (even very dilute ones) by certain types of inactive, dead, microbial biomass[6] .Some of the advantages of biosorption include competitive performance, heavy metal selectivity, cost-effectiveness, regenerative and no sludge generation. Sources of biomass include seaweeds, microorganisms (bacteria, fungi, yeast, and molds), activated sludge and fermentation waste. Studies using biosorbents have shown that both living and dead microbial cell are able to uptake metal ions and offer potential inexpensive alternative to conventional absorbents [7, 8]. However, living cell is subject to toxic effect of the heavy metals, resulting in cell death. Moreover, living cell often require the addition of nutrients and hence increase the BOD and COD in the effluent. For these reasons, the use of non-living biomaterials or dead cells as metal binding compounds has been gaining advantage because toxic ions do not affect them. In addition, dead require less care and maintenance, and cheaper [9] .Furthermore, dead biomass could be easily regenerated and reused. The capability of some living microorganisms to accumulate metallic elements has been observed at first from toxicological point of view [10, 11, and 12]. However, further researches have revealed that inactive/dead microbial biomass can passively bind metal ions via various physicochemical mechanisms. Therefore researches on biosorption have become an active field for the removal of metal ions or organic compounds. Biosorbent behavior for metallic ions is a function of the chemical make-up of the microbial cells of which it consists [13]. Mechanisms responsible for biosorption, although understood to a limited extent, may be one or combination of ion exchange, complexation, coordination, adsorption, electrostatic interaction, chelation and micro precipitation [14, 15 and 16].
Materials and methods
Biomass and culture medium
In this study chlorella pyrenoidosa (2738) obtained from National Collection of Industrial Microorganisms (NCIM) from PUNE – INDIA, which was isolated and thoroughly pure. The Chlorella pyrenoidosa was maintained in modified Fog,sMedia at 28 0C with using 3000 lx light intensity. After 21 days cultivation period cells were harvested by centrifugation and were washed several times with deionised water in order to remove culture media and was kept on a filter paper to reduce the water content. The biomass dried at 60 oC in an oven for 24 h and milled to a gritty consistency. The biomass was sieve for particle size smaller than 1 mm and stored in dark bottle and keeps in a dry cabinet for experiments. All of the media are sterilized by autoclaving at 121оC for 20 min.
Preparation of synthetic sample
A stock solution of 1000 mg/l of Ni was obtained by dissolving nickel chloride (Merck Company) in distilled water. The test solutions of various concentrations range from 10 mg/L to 100 mg/L were prepared from the stock solution. The solution pH was adjust using 0.1 M HNO3 and 0.1 M NaOH at the beginning of the experiment and not controlled afterwards. The conical flasks (250 mL) were shaken at 120 rpm in a temperature controlled rotatory shaker.
Analysis of Nickel ions
Nickel ions were determined spectrophotometrically by atomic adsorption spectrophotometer (UNICAM, model 929, UK).
Batch biosorption studies
Batch mode adsorption studies for individual metal compounds were carried out to investigate the effect of different parameters such as adsorbate concentration, adsorbent dose, agitation time and pH. Solution containing adsorbate and adsorbent was taken in 250 mL capacity conical flask and agitated at 120 rpm in a shaker at predetermined time intervals. The adsorbate was decanted and separated from the adsorbent using whatman No.41 filter paper.
Effect of agitation time and initial concentration
For the determination of rate of metal biosorption by biomasses from 100 ml (at 10, 20, 50, 100 mg/L) on conical flask 250 mL, the supernatant was analyzed for residual metal at different time intervals. The pH and the adsorbent dosage was kept constant at pH= 5± 0.01, which varied according to the adsorbent and adsorbate under consideration. Amount of biomass dosage was 0.1±0.001 g for biomass (Chlorella pyronoidsa) and temperature was 25 ±1 ◦C and agitation speed of shaker was 120 rpm.
Effect of adsorbent dosage and initial concentration
The effect of adsorbent dosage i.e., the amount of the biomasses on the adsorption of metals was studied at different dosages ranging from 0.1 to 3 g with varied metal concentrations of 10, 20 and 50 mg/L. The equilibrium time and the pH were kept constant depending on the metal under consideration. The pH and the adsorbent dosage was kept constant at pH= 5± 0.01, which varied according to the adsorbent and adsorbate under consideration. Agitation time was 120 minute for biomass (Chlorella pyronoidsa) and temperature was 25 ±1 ◦C and agitation speed of shaker was 120 rpm.
Effect of pH and initial concentration
To determine the effect of pH on the adsorption of metal solutions (100 mL) of different concentration ranges (10, 20 and 50 mg/L) at conical flask 250 mL were adjusted to desired pH values and mixed with constant amount of adsorbent and agitated at preset equilibrium time. The equilibrium time and adsorbent dosage varied with the metal and adsorbent under consideration. Amount of biomass dosage was 0.1±0.001 g for e biomass (Chlorella pyronoidsa) and temperature was 25 ±1 ◦C and agitation speed of shaker was 120 rpm and contact time was 120 min.
Effect of temperature
Optimum biomass concentration with optimum pH was used to monitor the temperature effect on biosorption. Experiments were carried out at different temperatures from 10-40 oC for each culture and kept on rotary shaker at 120 rpm. The samples were allowed to attain equilibrium. To determine the effect of temperature on the adsorption of metal solutions (100 mL) of concentration 50 mg/L at conical flask 250 mL were adjusted to desired pH values and mixed with constant amount of adsorbent and agitated at preset equilibrium time. The equilibrium time and adsorbent dosage varied with the metal and adsorbent under consideration. Amount of biomass dosage was 0.1±0.001 g for biomass (Chlorella) and pH was 5±0.001 and agitation speed of shaker was 120 rpm and contact time was 120 min.
Desorption studies
After adsorption, the adsorbates – loaded adsorbent were separated from the solution by centrifugation and the supernatant was drained out. The adsorbent was gently washed with water to remove any unadsorbed adsorbate. Regeneration of adsorbate from the adsorbate – laden adsorbent was carried out using the desorbing media – distilled water at pH ranges using dilute solutions of EDTA, HCL and HNO3 (Stirred at 120 rpm for 120 min at 25 0C) . Then they were agitated for the equilibrium time of respective adsorbate. The desorbed adsorbate in the solution was separated and analyzed for the residual heavy metals.
FT-IR spectroscopy (Fourier Transform Infrared)
In order to determine the functional groups responsible for Ni biosorption, IR spectroscopy was used that about 0.1 g biomass was mixed with KBr for FT-IR spectra analysis (Shimadzu model 8400).
SEM (Scanning Electron Microscopy)
The SEM was used to investigate the morphology of the biosorbent. We used samples with pH=5 and C0 = 10 mg.L-1. Scanning Electron Microscope (SEM, JEOL, JSM-6360A) used for this study.
Results
Results on the effect of pH of Ni at different initial metal ion concentrations by Chlorella pyrenoidosa present in the Figure 1. Maximum percentage of biosorption accursed at the initial concentration of 10 mg/L at the time of 120 minute at pH= 5 for Ni was 94.31% and metal ion uptake capacity was 9.43 mg/g and when initial concentration of Ni increased to 50 mg/L, percentage of biosortion of Ni was 86.60% and uptake capacity was 43.30 mg/g for Chlorella pyrenoidosa .With increase the initial concentration percentage of biosorption decreased and metal ion uptake capacity was increased. Results on the contact time of nickel at different initial metal ion concentrations by Chlorella pyrenoidosa present in the Figure 2. The time required to reach equilibrium for Ni adsorption by Chlorella pyrenoidosa was 60 minute for all initial metal ion concentrations. In the initial concentration of 10 mg/L at the time of 60 minute percentage of remove of Ni was 92.07% and metal ion uptake capacity was 9.27 mg/g and when initial concentration of Ni increased to 100 mg/L, percentage of remove of Ni was 63.34% and uptake capacity was 63.34 mg/g for Chlorella pyrenoidosa. The time taken for Ni adsorption by Chlorella pyrenoidosa was dependent on initial metal ion concentration and increased with increase in concentration of Ni. With increase the initial concentration percentage of biosorption decreased and metal ion uptake capacity was increased. Results on the effect of biomass dosage of Ni at different initial metal ion concentrations by Chlorella pyrenoidosa, present in the Figure 3. percentage of biosorption accursed at the initial concentration of 10 mg/L at the time of 120 minute at pH= 5 and 0.1± 0.001 g of biomass for Ni was 94.31% and metal ion uptake capacity was 9.43 mg/g and when initial concentration of Ni increased to 50 mg/L, percentage of biosortion of Ni was 86.60 % and uptake capacity was 43.30 mg/g for Chlorella pyrenoidosa. When amount of biomass increased from 0.1 ± 0.001 g to 3 ± 0.001 g, percentage of biosorption accursed at the initial concentration of 10 mg/L at the time of 120 minute at pH= 5 for Ni was 93.10 % and metal ion uptake capacity was 0.332 mg/g and when initial concentration of Ni increased to 50 mg/L , percentage of biosortion of Ni was 91.92 % and uptake capacity was 1.53 mg/g for Chlorella pyrenoidosa. With increase the initial concentration percentage of biosorption decreased and metal ion uptake capacity was increased. With increased the amount of biomass observed that percentage of biosorption increased and metal ion uptake capacity was decreased. Results on the effect of temperature of Ni at initial metal ion concentration of 50 mg/L by Chlorella pyrenoidosa present in the Figure 4. Maximum percentage of biosorption accursed at the initial concentration of 50 mg/L at the time of 120 minute at pH= 5 for Ni was 89.42% and metal ion uptake capacity was 44.71 mg/g at the temperature of 40 oC for Chlorella pyrenoidosa. The findings of Chlorella pyrenoidosa indicate that the sorption percentage increased with increase in temperature up to 40 o C.
Equilibrium isotherms
The isotherm studies were performed in the solution with the initial concentrations ranging from 10 to 100 mg/L at optimum pH values for ions (pH=4.5 or pH = 5) .After shaking the flask containing the mixture of biomass (120 rpm, 25 ºC) and ions for 120 min, the amount of residual ions in the filtrated solution was analyzed. The biosorption equilibrium uptake capacity for each sample was calculated according to mass balance on the ions expressed in this equation:
![]()
where V is the sample volume (L), C0 is the initial ion concentration (mg/L), Ce is the equilibrium or final ion concentration (mg/L (, M is the biomass dry weight (g), and qe is the biomass biosorption equilibrium ions uptake capacity (mg/g).Langmuir and Freundlich isotherms, the two classical adsorption models, were used to describe the equilibrium between adsorbed ions on the biomass cell (qe ,q) and ions in the solution (Ce, q) in this study.
Langmuir isotherm model:
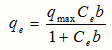
That after arrange we have;
![]()
These values qmax and b (where b, is the adsorption equilibrium constant) can be obtained from the slopes and the intercepts of the linear plots respectively, where experimental data of Ce/qe as the function of Ce. .The empirical Freundlich equation based on sorption on a heterogeneous surface, on the other hand, is as follows:
qe = Kf (Ce) n
K and n: An experimental constant, K is an indication of the adsorption capacity of the adsorbent; n indicates the effect of concentration on the adsorption capacity and represents adsorption intensity. The equation can be linearized in the following logarithmic form:
![]()
These values n & Kf can be obtained from the slopes and the intercepts of the linear plots respectively, where experimental data of Ln qe as the function of Ln Ce. .The equilibrium experimental results of Nickel ions have been fitted in the Langmuir and Freundlich models. For biosorption of Nickel using chlorella pyrenoidosa the coefficient of determination (R2) of both models was mostly close to 1 as shown in Figure 6. This indicates that both models adequately describe the experimental data of the biosorption of Nickel. In the biosorption of Nickel by chlorella, most of the metal ions were sequestered very fast from the solutions in the first phase of contact time 60 minutes and almost no increase in the level of bound metal have been occurred after this time interval. Biosorption equilibrium isotherms were plotted for metal uptake q against the residual metal concentration in the solution. The q verses Cf sorption isotherm relationship was mathematically expressed by Langmuir and Freundlich models. The higher the values of k and n; lower the value of b, the higher the affinity of the biomass. Table1 describes summaries of linear regression data for Langmuir and Freundlich isotherms for Nickel biosorption using chlorella pyrenoidosa biomass. Langmuir and Freundlich constant k were obtained from the linear equations of both models. As indicated in the Table 1, the coefficients of determination (R2) of both models are close to 1. In the Table 1 the values of Kf, n, q max and b were given.
Kinetic modeling
the experimental break through curves for the effects of contact time on a bound rate of Ni. It can be observed that the adsorption of Nickel ions quickly increased at the beginning of biosorption, but after 15 min, the adsorption slowed down. The result indicated that the maximum adsorbed amount of the Nickel ions was achieved within 60min, and then followed by a longer equilibrium period. After this equilibrium period, the amount of adsorbed ions did not significantly change with the adsorption time. Therefore, for the following experiments, the contact time was maintained for 60 min to ensure that equilibrium was fully achieved.
The pseudo-second-order equation is also based on the sorption capacity, which is expressed as:
![]()
Where K2 is the rate constant of pseudo-second-order sorption (g· mg-1·min-1). K2qe2 is the initial rate constant (represented by h, mg·g−1·min−1). Plotting t/qt versus t will give a straight line. The values of qe and K2 can be determined from the slope and intercept of the plot, respectively. The results showed that the pseudo-second-order model fitted the simulation curve much better than the pseudo-first-order model for Ni. The results of pseudo-second-order model showed on the Table 2.The coefficient of determination (R2) and K2 HO,S Model for the different metal ion concentration under study for has been established as:10 ppm > 20 ppm > 50 ppm > 100 ppm
With increase the initial concentration coefficient of determination (R2) and K2 decreased.
Equilibrium parameter RL
The essential characteristics of a Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter RL, which is defined by the equeation : RL = 1/1+bCo
Where Co is the initial adsorbate concentration (mg/L) and b is the Langmuir constant (L/mg). The parameter indicates the shape of the isotherm as follows (Table3).The RL values at different initial adsorbate concentrations indicate favorable adsorption for all the adsorbents and adsorbates studied.
Thermodynamic studies
Adsorption, studies in the temperature range 283–313 K were conducted to determine thermodynamics constants such as Gibbs free energy change (∆G°), enthalpy change (∆H°) and entropy change (∆S°) for the system and to ascertain the sorption mechanism. For this study, adsorbent dosage selected was 0.1 gr. and Nickel concentration was 50 mg L-1 with pH= 5 in a conical flask and allowed to equilibrate for 1 h at the different temperatures ranging from 283 to 313 K. In order to determine thermodynamic parameters, experiments were carried out at different temperatures in the range of 283–313 K for heavy metals adsorption. The thermodynamic parameters such as standard Gibbs free energy change (∆G°), enthalpy change (∆H°) and entropy change (∆S°) were estimated to evaluate the feasibility and nature of the adsorption process. The Gibbs free energy change, of the process is related to equilibrium constant by the equation:
where, T is temperature in K, R is ideal gas constant having value as 8.314, J mol-1 K-1 and KC is thermodynamic equilibrium constant. The thermodynamic equilibrium constant, was determined as:
Where, Ca is mg of adsorbent adsorbed per liter and Ce is the equilibrium concentration of solution, mg/L. According to thermodynamics, the Gibbs free energy change is also related to the enthalpy change (∆H°) and entropy change (∆S°) at constant temperature by the following Van, t Hoff equation:
In Kc=![]()
The values of enthalpy change (∆H°) and entropy change (∆S°) were calculated from the slope and intercept of the plot lnKc versus, 1/T. Results on the Plots of Van, t Hoff equation for the estimation of thermodynamic parameters by Chlorella pyrenoidosa present in the Figures 8.The calculated values of thermodynamic parameters are reported in Table 4.A positive value of ∆H° indicated the endothermic nature of the process. A negative value of the free energy (∆G°) indicated the spontaneous nature of the adsorption process. It was also noted that the change in free energy, increases with increase in temperature, which exhibits an increase in adsorption with rise in temperature. This could be possibly because of activation of more sites on the surface of biomasses with increase in temperature. A positive value of ∆S° showed increased randomness at solid–liquid interface during the adsorption of heavy metals, it also suggests some structural changes in the adsorbate and the adsorbent.
FRIR Spectroscopy of Chlorella pyrenoidosa
Spectrums of Chlorella pyrenoidosa present in the Figure 9 that revealed the presence of hydroxyl, amino, carboxylic and carbonyl groups. The presence of OH group along with carbonyl group confirmed the presence of carboxylic acid groups in the biosorbent. The presence of NH group and OH group along with carbonyl group might be attributed the presence of amino acid groups in the biosorbent.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) of Chlorella pyrenoidosa at before and after biosorption of Nickelpresent in the Figures 10 and 11 respectively. The scanning electron micrograph clearly revealed the surface texture and morphology of the biosorbent at different magnifications. The SEM analysis revealed important information on surface morphology. In these micrographs structures with large surface area were evident.
Desorption studies
Desorption and regeneration studies of the adsorbates showed that regeneration and recovery of the adsorbates is possible. Chemisorption/ion exchange was the main mechanism by which the adsorbates (metals) were attached to the adsorbents. Physical adsorption played a minimal role in the process .The result of desorption studies of Ni in a batch system showed that HNO3 (0.1 M) was more efficient in Ni desorption, which remove 97% Nickel ions (Table 5).
Conclusions
The batch experiment conducted with the biosorption demonstrated that biomass of chlorella pyrenoidosa exhibited the potential for Ni removal from aqueous solution. Optimum pH and temperature for biosorption in this study were 5 and 25 oC, respectively. The time taken for Ni adsorption by chlorella pyrenoidosa was dependent on initial metal ion concentration and increased with increase in concentration of Ni. With increase the initial concentration percentage of biosorption decreased and metal ion uptake capacity was increased. With increased the amount of biomass observed that percentage of biosorption increased and metal ion uptake capacity was decreased. The findings of chlorella pyrenoidosa indicate that the sorption percentage increased with increase in temperature up to 30 o C and there was a decrease in sorption percentage with further increase in temperature. The removal of Ni increase with increase in biosorbent .The biosorption process was followed both Langmuir and Freundlich isotherm model. The pseudo second-order kinetics described the experimental data well. The equilibrium time was 60 min. The RL values at different initial adsorbate concentrations indicate favorable (0<RL<1) adsorption for all the adsorbents and adsorbates studied.HNO3 (0.1M) had higher efficiency of Ni desorption than EDTA (0.1M) and HCL(0.1M) with 95% efficiency desorption.A positive value of ∆H° indicated the endothermic nature of the process. A negative value of the free energy (∆G°) indicated the spontaneous nature of the adsorption process. A positive value of ∆S° showed increased randomness at solid–liquid interface during the adsorption of heavy metals, it also suggests some structural changes in the adsorbate and the adsorbent. FTIR Spectrums of Chlorella pyrenoidosa revealed the presence of hydroxyl, amino, carboxylic and carbonyl groups. The scanning electron micrograph (SEM) clearly revealed the surface texture and morphology of the biosorbent.
References
- Sitting M., Toxic Metals—Pollution Control and Worker Protection, Noyes Data Corporation, New Jersey 1976.
- Al-Asheh S. and Duvnjak Z., Sorption of cadmium and other heavy metals by pine bark, Adv. Environ. Res. 1997, 1 ,194.
- Kadirvelu K., Preparation and characterization of activated carbon, from coir pith and its application to metal bearing wastewater, Ph.D. Thesis, Bharathiar University, Coimbatore, India, 1998.
- Beliles R.P., The lesser metals. In: F.W. Oehme, Editor, Toxicity of Heavy Metals in the Environment, Part 2, Marcel Dekker, New York 1979, 383.
- Ahalya N, Ramachandra T.V., Kanamadi R.D , Biosorption of heavy metals, Journal of Chemistry and Environment, 2003, 7(4): 71-79.
- Macaskie, L.E.; Empson, R.M.; Cheetham, A.K.; Grey, C.P.; Skarnulis, A.J. Science 1992, 257, 782-784.
- Khoo K. M. & Ting Y. P., Biosorption of gold by immobilized fungal biomass, Biochem Eng. J., 2001, 8, 51-59
- Knorr D, Recovery and utilization of chitin and chitosan in food processing waste management, Food Technol, 1991, 45, 114-122.
- Mofa A S, Plants proving their worth in toxic metal cleanup, Science, 1955, 269,302-305.
- Volesky B. Biosorption by fungal biomass. In: Volesky B, editor. Biosorption of heavy metals. Florida: CRC press; 1990 a. 139–71.
- Volesky B. Introduction. In: Volesky B, editor. Biosorption of heavy metals. Boca Raton: CRC press; 1990 b. 3–5.
- Volesky B. Removal and recovery of heavy metals by biosorption. In: Volesky B, editor.Biosorption of heavy metals. Florida: CRC press; 1990 c. 8-43.
- Volesky B, Holan ZR. Biosorption of heavy metals. Biotechnol Prog 1995; 11:235–50.
- Veglio F, Beolchini F. Removal of metals by biosorption: a review. Hydrometallurgy 1997; 44:301–16.
- Vijayaraghavan K, Yun YS. Bacterial Biosorbents and biosorption. Biotechnol Adv 2008; 26:266–91.
- Wang JL, Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: a review.Biotechnol Adv 2006; 24:427–51.

This work is licensed under a Creative Commons Attribution 4.0 International License.









