Study of Binary and Ternary Metal Complex Formation by Peptides by Solution Electrophoresis in Solution [Cu (Ii)/Ni (Ii)/Co (Ii)/Zn (Ii) - Glycyl Sarcosine -Nta-System]
Manorama Singh, Amar Kant and Satyendra Singh
Chemical Laboratory, Kashi Naresh Govt. Post Graduate College, Gyanpur, Sant Ravi Das Nagar, Bhadohi - 221 304, India.
An innovative solution electrophoresis technique has been used for the study of mixed complexes of some divalent metals ions viz. Cu (II), Ni (II), Co(II) and Zn(II). with Glycyl Sarcosine as primary ligand and NTA as secondary ligand.. The stability constants of the mixed complexes formed were found to be : 6.64, 4.85, 4.53, 4.42 (Log k values) for Cu (II), Ni (II), Co(II) and Zn(II) respectively at 30°C an ionic strength 0.1 M.
KEYWORDS:Solution Electrophoresis; Stability constants; Glycyl Sarcosine; Mixed complexes.
Download this article as:| Copy the following to cite this article: Singh M, Kant A, Singh S. Study of Binary and Ternary Metal Complex Formation by Peptides by Solution Electrophoresis in Solution [Cu (Ii)/Ni (Ii)/Co (Ii)/Zn (Ii) - Glycyl Sarcosine -Nta-System]. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Singh M, Kant A, Singh S. Study of Binary and Ternary Metal Complex Formation by Peptides by Solution Electrophoresis in Solution [Cu (Ii)/Ni (Ii)/Co (Ii)/Zn (Ii) - Glycyl Sarcosine -Nta-System]. Available from: http://www.orientjchem.org/?p=24724 |
Introduction
For the study of Metal-Ligand equilibria partition technique, solvent extraction, ion exchange method, and paper electropheresis have been mainly employed by a number of workers. Jokl [1] has done a significant work for the determination of stability constants of Metal complexes adopting the electro migration studies. From the migration mobility curve, he succeeded in determining the stability constants of amino acid complex of some bivalent metal ions. A theoretical treatment was given by Biernet [2] for the study of stepwise complex formation. The technique subsequently attracted the attention of a few workers [3-5] who applied it to examine various complexing system in a aqueous medium.
In recent years, Singh et. al. have published a number of paper in which a new approach have been made for the study of complexation reaction in solution with the help of paper electrohoresis [6-12].
The gel or paper electrophoresis has the striking drawback in the sense that the path of migrating ion is not uniform. The surface of paper of gel medium, on which the charged speices moves, deponds on the mode of manufacturer of the paper of gel. Keeping the discrepancies in mind, a venture to work in pure solution in this paper has been under taken. According to Glasstone[13], “relatively little work has been done on the transference number of ions in mixtures, although, both Hittorf and moving boundary methods have been employed. It is possible, to derive the required transference numbers by the analysis of the anodic and cathodic compartments before and after electrophoresis.
In the present work Glycyl Sarcosine as primary ligand and nta as secondary ligand has been studied from the point of the view of the complexation with four metal ions viz., Cu (ii), Ni (ii), co (ii), zn (ii).
Experimental
Instruments
Electrophoretic tube
A simple Electrophoretic tube, 18 cm long and of 5 mm bore with a stopper in middle and is fused perpendicularly at the ends with short wider tubes of 1.2 cm bore, arms have been utilized to insert the platinum electrodes. These electrodes are connected with an Electrophoresis voltage supply. The voltage can be varied through three different ranges viz. 0 – 100, 100 – 200, and 200 – 300 volts.
pH – Indicator and Accessories
CP901 Century digital pH – meter having glass electrode assembly and working on 220 volts / 50 cycles stabilized A.C. main was used.
Colorimeter
A colorimeter of visible range 400 – 750 nm of carlzeiss (Jena Specol) was employed.
Chemicals
Cu (II), Ni (II), Co (II), Zn (II) Perchlorate Solutions were prepared by precipitating the corresponding carbonates from 0.1 M solution of sulphates of metal with solution of sodium carbonate, washing the Precipitates with water and treated with AR grade 1% Perchloric Acid. These were boiled on a water bath and filtered to get stock solution of the Metal Perchlorate 5.0 × 10-3 M (Approx)
Stock solution of the complexing reagents Glycyl Sarcosine were prepared by dissolving accurately weighted amounts in water. Solutions of required strengths were then prepared by suitable dilutions.
Perchloric acid as background electrolyte :
A stock solution (1.0 M) was prepared by suitable dilution of 70% Perchloric Acid. The solution was standarised by titrating a suitable volume of its dilute solution against a standard NaOH solution.
Detecting Reagent for Cu(II), Ni(II), Co(II) and Zn (II)
Ammonim Thiocynate Solution for Cu (II), Dimethyl Glyoxime for Ni (II)k, Stannous Chloride solution, ammonium thiocynate and acetone for Co (II), Zincon [5-(2-Corboxy-phenyl] – 1-(2-Hydroxy 5-sulpho phenyl formazon] for Zn (II)[14].
Procedure
At the outset a solution containing 1.0 × 10–2 M and Glycyl Sarcosine , 0.1 M Perchloric Acid solution and respective amount of metal ion solution [2.0 × 10–3 Cu (II), 2.0 × 10–3 Ni (II) or 1 × 10–4 Co (II), and Zn (II) were prepared Respectively. The pH of the solution was adjusted by adding sodium hydroxide solution. An Aliquot of 10 ml ion taken in the electrophoretic tube and then thermostated at 30ºC. After allowing electrolysis 30 minutes, the middle stopper was closed and developing the solution of anodic Compartment by adding developers. The absorbance of the solution was taken at lmax 625 nm respectively.
The observed mobility of migrating cation was calculated by measuring the change in the absorbance of the solution contained in anodic compartment.
Firstly the absorbance taken before electrolysis (A0) and the after passing electricity for 30 minutes at potential diff 50V, the middle stopper was closed. This was At. The difference between these two give the mobility of respective Ion. Under a potential gradient, a metal ion will more in the field, the speed and its direction depending upon the charges and size of the ion.
Result and Discussion
M (II) – Glycyl Sarcosine binary system
The plot of the overall mobility of a metal spot against pH gives a curve with a number of plateaus. The first, at the beginning, corresponds to a region in which metal ions are uncomplexed. A second plateau in each instance with positive mobility indicates the formation of 1: 1 complex of a cationic nature. A further increase of pH results in a third plateau with zero mobility, which indicates the formation of an electrically neutral metal complex. The literature also assigns prominent liganding properties to unprotonated anionic species of Glycyl Sarcosine , ruling out any such property to the zwitterion[6]. In view of the above observation, the complexation of a metal ion with the Glycyl Sarcosine anion L– may be represented by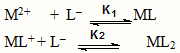
The metal spot on the paper is thus a conglomeration of uncomplexed metal ion and 1:1 and 1:2 complexes. The overall mobility, U, is given by
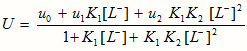
where u0, u1 and u2 are the mobilities of the uncomplexed metal ion, 1:1 complex and 1:2 complex, respectively.
For calculating the first stability constant, K1 , the region between the first and second plateau is pertinent. The overall mobility U will be equal to the arithmetic mean of the mobility of the uncomplexed metal ion, u0, and that of the first complex, u1, at a pH where K1 = 1/[L–] with the help of dissociation constants of Glycyl Sarcosine (k1 = 103.34,
k1 = 1010.36) [16,17].
The concentration of liganding Glycyl Sarcosine , L–, is calculated with the equation
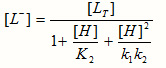
where [LT] = total concentration.
The stability constant K2 of the second complex can be calculated by taking into consideration the region between the second and third plateaus of the mobility curve. These calculated values are given in Table I.
Metal-NTA system :
The absorbance difference of metal ion solution in presence of NTA at different pH are plotted. The absorbance difference of last plateau in case of Cu (II), Ni (II), Co (II) and Zn (II) is negative. Hence this indicate anionic nature of metal NTA complex. Hence only one NTA anion to combine with metal ion to give 1:1 complexes. The stability constant of complexes with NTA were calculated as described in metal penicillamine complexes and is given in Table-1.
M-Glycyl Sarcosine -NTA-mixed Complexes :
The study of this system was made at pH 8.5. From the absorbance difference Vs. pH curves for metal- Glycyl Sarcosine and Metal-NTA binary complex system that binary complexes are form at pH 8.5. Hence it was considered appropriate to study the transformation of ML2 to ML-NTA at pH 8.5 in order to avoid any side interaction. The study of these mixed complexes have been carried out in presence of Glycyl Sarcosine with progressive addition of secondary ligand NTA from 1 × 10-7 M to 5 × 10-3 M at a fixed pH 8.5. The observations are plotted. These figures elucidate the transformation, of ML2 in to M-L-NTA complexes on progressive addition of NTA. The figure shows two plateaus. The first plateau corresponds to M-(Glycyl Sarcosine )2 whereas the second plateau corresponds to a new complex. This new complex may be a binary complex M-NTA produced in accordance with the interaction, where the legend L is completely replaced by the NTA.
The new complex may also be a mixed complex of M – L – NTA as M –L2+ + NTA ® M – L – NTA + L in which the NTA adds on to ML giving an anionic species.
Obviously the final plateau corresponds to the absorbance difference of M – NTA or M – L – NTA, whichever is formed, in interaction. It was found that the absorbance difference of the new species formed is not identical to the absorbance difference of M – NTA (binary complex) as observed in pure metal ion and NTA interaction. The new absorbance is greater in magnitude than that of M – NTA. This confirms the formation of M – L – NTA Complex. The between the two plateaus represent the progressive transformation of binary complex ML2 into ML–NTA mixed complex as
![]()
The K’ can be calculated with the help of the method of mean mobility obviously K’ will be given by reciprocal of the tri negative anion concentration of NTA at the mid point of two plateaus. The calculated value of stability constant are given in table-1.
Table 1: Stability Constants of Some Binary and Ternary Complexes of
Cu (II), Ni (II), Co (II) and Zn (II)
Ionic strength, m = 0.1 ; temperature = 30ºC.
|
Metal Ion |
Calculated value of stability constantsa |
|||
|
Log
|
Log |
Log
|
Log
|
|
|
Cu (II) |
6.64 |
17.97 |
12.24 |
6.64 |
|
Ni (II) |
4.85 |
12.73 |
10.81 |
4.85 |
|
Co (II) |
4.53 |
12.45 |
10.32 |
4.53 |
|
Zn (II) |
4.40 |
12.36 |
10.59 |
4.42 |
![]()
References
- Jokl, V., J. Chromatography, 14, 71, (1964).
- Biernet, J., Rocz. Chem., 38, 343, (1964).
- Hurnick, B., Rocz, Chem., 39, 137 (1965).
- Koch, H. & Lovchev, M., Isotopanpraxis, 7, 401 (9171).
- Kozak, J., Acta fae. Perum Nature Univ. Comenianeae, Chem. 23 (1971).
- Singh, S., Yadav, K.L., Annli Di Chimica, 75, 377 (1985).
- Tiwari B.B. and Yadav, K.L.; Trans. Saest., 25(4), 124 (1990).
- Tewari B.B. and Yadav, K.L., J. Chromatogr, 542-537 (1991).
- Tewari B.B. and Yadav, K.L., Biomed. Chromatogr, 10 (5), 221 (1996).
- Tewari B.B., J. Chromatogr, 910 (1), 181 (2001).
- Singh, S., Bajpai, A.K. and Yadav, K.L., Electrophoresis, 7, 187(1986).
- Singh, S., Gupta, D. and Yadav, K.L., J. Electrochem. Soc., 35, 1(1986).
- Glasstone, S. Electrochemistry (2001).
- Wagner, W., Hull, C.J., and Markle, G.E., Advanced Analytical Chemistry Reinhold Publishing Corporation, New York (1956).

This work is licensed under a Creative Commons Attribution 4.0 International License.









