Spectral Investigation of the Activities of Amino Substituted Bases
Muhammad Aqeel Ashraf1, Abdul Wajid2, Karamat Mahmood2, Mohd.Jamil Maah1 and Ismail Yusoff3
¹Department of Chemistry, University of Malaya, 50603, Kuala Lumpur (Malaysia). ²Department of Chemistry, The Islamia University of Bahawalpur - 63100 (Pakistan). ³Department of Geology, University of Malaya, 50603, Kuala Lumpur (Malaysia).
Three new series of biologically active amino substituted Schiff bases with general formula, R1 N=CHR2 . Here R1 = 2-amino-benzthiazole, 4-amino-salicylic acid and 4-aminophenol. R2 =4-chlorobenzaldehyde, 2-chloro-benzaldehyde, salicylaldehyde, vanillin and benzaldehyde were synthesized by the reaction of three different amino substituted compounds and substituted aldehydes in ethanol. Such compounds were characterized by different physico-chemical techniques like, melting point, elemental analysis, multinuclear nmr (1 H, 13C). The free ligands and their metal complexes have been screened for their in vitro biological activities against bacteria, fungi and yeast. The metal complexes show more potent activities compared with Schiff base ligands.
KEYWORDS:Schiff bases; Benzthiozol; Aminophenol; Antibacterial; and Antifungal activity
Download this article as:| Copy the following to cite this article: Ashraf M. A, Wajid A, Mahmood K, Maah M. J, Yusoff I. Spectral Investigation of the Activities of Amino Substituted Bases. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Ashraf M. A, Wajid A, Mahmood K, Maah M. J, Yusoff I. Spectral Investigation of the Activities of Amino Substituted Bases. Available from: http://www.orientjchem.org/?p=11666 |
Introduction
Schiff bases derived from aromatic amines and aromatic aldehydes have a wide variety of applications in many fields, e.g., biological, inorganic and analytical chemistry [1-5]. Application of many new analytical devices requires the presence of organic reagents as essential compounds of the measuring system. They are used, e.g., in optical and electrochemical sensors, as well as in various chromatographic methods, to enable detection of enhance selectivity and sensitivity [6-8].Among the organic reagents actually used, Schiff bases possess excellent characteristics, structural similarities with natural biological substances, relatively simple preparation procedures and the synthetic flexibility that enables design of suitable structural properties [9,10]. Schiff bases are widely applicable in analytical determination, using reactions of condensation of primary amines and carbonyl compounds in which the azomethine bond is formed (determination of compounds with an amino or carbonyl group); using complex formation reactions (determination of amines, carbonyl compounds and metal ions); or utilizing the variation in their spectroscopic characteristics following changes in pH and solvent (pH of solvent polarity indicators) [1,11-13]. Unfortunately, most Schiff bases are chemically unstable and show a tendency to be involved in various equilibria, like tautomeric interconversions, hydrolysis, or formation of ionized species [14, 15]. Therefore, successful application of Schiff bases requires a careful study of their characteristics.
Schiff bases resulted from aromatic aldehydes ortho-substituted with a hydroxyl group have initially arouse the researchers’ interest because of their ability to act as bidentate ligands for transitional metal ions [16-20]. Later, in studies concerning quantitative structure-antitumor activity relationship of a series of Schiff bases derived from variously substituted aromatic amines and aldehydes, it has been shown that azomethines from salicylaldehydes gave the best correlation [21, 22]. Schiff bases of salicylaldehydes have also been reported as plant growth regulators [23] and antimicrobian [24] or antimycotic [25] activity. Schiff bases also show some analytical applications [26]. Schiff Bases are characterized by the -N=CH- (imine) group which imports in elucidating the mechanism of transamination and rasemination reaction in biological system [27, 28]. Schiff bases are active against a wide range of organisms for example; Candida Albicans, Escherichia coli Staphylococcus aureus, Bacillus polymxa, Trychophyton gypseum, Mycobacteria, Erysiphe graminis and Plasmopora viticola.
A large number of different Schiff base ligands have been used as cation carriers in potentiometric sensors as they have shown excellent selectivity, sensitivity, and stability for specific metal ions such as Ag(II), Al(III), Co(II), Cu(II), Gd(III), Hg(II), Ni(II), Pb(II), Y(III), and Zn(II) [29-34]. Schiff bases have been studied for their important properties in catalysis [35]. They show catalytic activity in hydrogenation of olefins [36]. They find applications in biomimetic catalytic reactions.
An interesting application of Schiff bases is their use as an effective corrosion inhibitor, which is based on their ability to spontaneously form a monolayer on the surface to be protected. Many commercial inhibitors include aldehydes or amines, but presumably due to the C=N bond the Schiff bases function more efficiently in many cases [37]. The principal interaction between the inhibitor and the metal surface is chemisorption [38]. The inhibitor molecule should have centers capable of forming bonds with the metal surface by electron transfer. In such cases the metal acts as an electrophile and the inhibitor acts as a Lewis base. Nucleophilic centers, such as oxygen and nitrogen atoms, of the protective compound have free electron pairs which are readily available for sharing. Together with the atoms of the benzene rings they create multiple absorption sites for the inhibitor thus enabling stable monolayer formation [39].
Imines also have biological importance. An imine linkage between the aldehyde derived from vitamin A and the protein opsin in the retina of the eye plays an important role in the chemistry of vision. Vitamins are also called coenzymes, meaning that they are to the functioning of many enzymes, which are large proteins that catalyze chemical changes in cell. An example of a biologically important aldehyde is pyridoxal phosphate, which is the active form of the vitamin B6. Vitamin B6 serves as a coenzyme by forming an imine with an amino acid grouping an enzyme. The coenzyme, bound to the enzyme, is involved in transamination reaction, the transfer of the amino group from one amino acid to another, which is important in the metabolism and the biosynthesis of amino acids. In the last step, enzyme-catalyzed hydrolysis cleaves the imine to pyridoxal and the modified amino acid.
Schiff bases have been reported in their biological properties, such as, antibacterial, antifungal activities [40-43]. Their metal complexes have been widely studied because they have anticancer and herbicidal applications [44, 45]. They serve as modals for biologically important species.
o-phenylenediamine Schiff bases shows clinical properties [46]. Isatin Schiff bases were reported to possess antiviral, anti-HIV, antiprotozoal and anthelmintic activities [47]. They also exhibit significant anticonvulsant activity, apart from other pharmacological properties [48]. Certain cobalt Schiff base complexes are potent antiviral agents [49]. Schiff bases derived from 4-dimethylamine benzaldehyde shows antibacterial activity. In medicines used as antibodies and anti-inflammatory agents [50-54]
This paper presents a series of new Schiff bases with a potential biological activity resulted from the acid-catalyzed condensation of aryl aldehydes with aromatic and heteroaromatic amines. These compounds could also act as valuable ligands. The structures of the Schiff bases synthesized from 2-amino-Benzthiazole, 4-amino-Salicylic acid and 4-aminophenol are shown in Scheme 1:
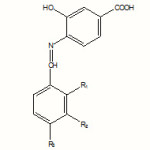 |
Scheme 1 Click here to View Scheme |
Material and Methods
Schiff bases’ melting points were taken on a Stuart Melting point apparatus SMP-3and are uncorrected. Elemental analysis was carried out at Fisons EA 1108 CHNSO Micro analyzer, lH and 13C NMR spectra were determined in DMSO (internal standard TMS) on Bruker spectrometer.
2-amino-benzthiazole, 4-amino-salicylic acid, 4-aminophenol, 4-chloro-benzaldehyde, 2-chloro-benzaldehyde, Salicylaldehyde, Vanillin, Benzaldehyde were purchased from Fluka and used without further purification. All organic solvents were purchased from Merck.
Synthesis of 2-amino-benzthiazole Schiff Bases
2g of 2-amino-benzthiazole was mixed with equivalent amount of corresponding aldehyde in 25 ml of ethanol. The resulting mixture was left under reflux for 2 h and the solid product formed was separated by filtration, purified by recrystallization from ethanol, washed with ethanol, and then dried.
Synthesis of 4-amino-salicylic acid Schiff Bases
2g of 4-amino-salicylic acid was mixed with equivalent amount of corresponding aldehyde in 25 ml of ethanol. The resulting mixture was left under reflux for 2 h and the solid product formed was separated by filtration, purified by recrystallization from ethanol, washed with ethanol, and then dried.
Synthesis of 4-aminophenol Schiff Bases
2g of 4-aminophenol was mixed with equivalent amount of corresponding aldehydes in 25 ml of ethanol. The resulting mixture was left under reflux for 2 h and the solid product formed was separated by filtration, purified by recrystallization from ethanol, washed with ethanol, and then dried.
Biological Activity
The synthesized Schiff bases were screened for antibacterial and antifungal activity.
Antibacterial Testing
The bacterial cultures for B. subtilis, S. aureus, and E. coli were obtained from Department of Microbiology University of Malaya, Kuala Lumpur, Malaysia. The bacterial cultures were incubated at 30 ± 0.1oC for 24 hours by inoculation into nutrient agar. Schiff bases were stored dry at room temperature and dissolved 20mg/ml in dimethylsulfoxide (DMSO). Antibacterial activities of each compound were evaluated by the agar disc-diffusion method. Mueller Hinton Agar Media (15 cm3) kept at 45oC was poured in the petri-dishes and allowed to solidify. Poured Petri plates (9 cm) were incubated with 50µL of normal saline solution of above culture media (105-106 bacteria per ml). Discs injected with prepared Schiff bases (50µL) were applied on the solid agar medium by pressing tightly. The Petri plates were placed at 37oC for 24 hours. At the end of period the inhibition zones formed on media were measured with a zone reader in millimeters.
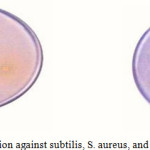 |
Figure 1: zone of inhabitation against subtilis, S. aureus, and E. col Click here to View figure |
Antifungal Testing
Pathogenic strains of Asperigillus niger and Chalara corda were obtained from Department of Microbiology University of Malaya, Kuala Lumpur, Malaysia. Schiff bases were stored dry at room temperature and dissolved 20mg/ml in dimethylsulfoxide (DMSO). Antifungal activities of each compound were evaluated by the agar disc-diffusion method. Sabarod’s agar media (15 cm3) kept at 45oC was poured in the petri-dishes and allowed to solidify. Sterile, filter paper discs of 10mm diameter were impregnated with prepared Schiff bases (50µL) and were placed on to the media, seeded with fungus. The plates were then incubated at 27oC for 1-7 days. At the end of period the inhibition zones formed on media were measured with a zone reader in millimeters.
Results and Discussion
Scheme 1
Twelve new Schiff bases have been synthesized from the condensation of 2-amino-Benzthiazole, 4-amino-Salicylic acid and 4-aminophenol with 4-chloro-benzaldehyde, 2-chloro-benzaldehyde, salicylaldehyde, vanillin and benzaldehyde (Scheme 2, 3, 4). The analytical and physical data are listed in Table 1.
Table 1: Physical data of Schiff Bases with general formula R1N=CHR2.
|
Comp no. |
R1 |
R2 |
Molecular Formula |
Melting Point oC |
Yield (%) |
Physical state |
Solubility |
Elemental Analysis |
|||
|
% C (Found) |
% H (Found) |
% N (Found) |
% S (Found) |
||||||||
|
1 |
2-amino-benzthiazole |
4-chloro-benzaldehyde |
C14H9ClN2S |
123-126 |
63 |
Light yellow Solid |
CHCl3, C2H5OH, DMSO |
61.65 (61.71) |
3.30 (3.32) |
10.27 (10.41) |
11.74 (11.87) |
|
2 |
2-amino-benzthiazole |
2-chloro-benzaldehyde |
C14H9ClN2S |
176-181 |
66 |
Light yellow Solid |
CHCl3, C2H5OH, DMSO |
61.65 (62.05) |
3.30 (3.41) |
10.27 (10.63) |
11.74 (11.92) |
|
3 |
2-amino-benzthiazole |
Salicylaldehyde |
C14H10N2OS |
145-150 |
72 |
Orange Solid |
CHCl3, C2H5OH, DMSO |
66.14 (65.94) |
3.93 (4.15) |
11.02 (11.45) |
12.59 (12.61) |
|
4 |
2-amino-benzthiazole |
Benzaldehyde |
C14H10N2S |
119-125 |
69 |
Light yellow Solid |
CHCl3, C2H5OH, DMSO |
70.59 (71.46) |
4.20 (4.41) |
11.76 (11.89) |
13.44 (13.51) |
|
5 |
2-amino-benzthiazole |
Vanillin |
C15H12N2O2S |
– |
59 |
Yellow Liquid |
CHCl3, C2H5OH, DMSO |
63.38 (63.89) |
4.22 (4.47) |
9.85 (9.97) |
11.26 (11.78) |
|
6 |
4-amino-salicylic acid |
2-chloro-benzaldehyde |
C14H10ClNO3 |
140-223 |
61 |
Brown Solid |
C2H5OH, DMSO |
60.98 (61.21) |
3.62 (3.71) |
5.08 (5.21) |
– |
|
7 |
4-amino-salicylic acid |
Salicylaldehyde |
C14H11NO4 |
181 |
74 |
Brick Red Solid |
DMSO |
65.36 (64.69) |
4.28 (4.41) |
5.44 (5.47) |
– |
|
8 |
4-aminophenol |
4-chloro-benzaldehyde |
C13H10ClNO |
184-190 |
62 |
Brown Crystalline Solid |
C2H5OH, DMSO |
67.38 (67.36) |
4.31 (4.30) |
6.04 (6.01) |
– |
|
9 |
4-aminophenol |
2-chloro-benzaldehyde |
C13H10ClNO |
150-159 |
67 |
Brown Crystalline Solid |
C2H5OH, DMSO |
67.38 (67.36) |
4.31 (4.30) |
6.04 (6.01) |
– |
|
10 |
4-aminophenol |
Salicylaldehyde |
C13H11NO2 |
142-155 |
74 |
Reddish Crystalline Solid |
C2H5OH, DMSO, CHCl3 |
73.23 (73.22) |
5.16 (5.15) |
6.57 (6.55) |
– |
|
11 |
4-aminophenol |
Vanillin |
C14H12ClNO2 |
208-215 |
57 |
Brown Crystalline Solid |
C2H5OH, DMSO |
69.13 (69.12) |
5.34 (5.32) |
5.76 (5.75) |
– |
|
12 |
4-aminophenol |
Benzaldehyde |
C13H11NO |
187-192 |
64 |
Pale yellow Crystalline solid |
C2H5OH, DMSO |
79.18 (79.16) |
5.58 (5.54) |
7.10 (7.13) |
– |
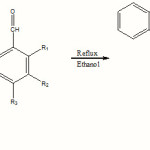 |
Scheme 1 Click here to View scheme |
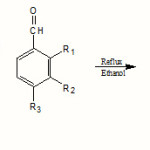 |
Scheme 2 Click here to View scheme |
 |
Scheme 3 Click here to View scheme |
NMR Spectroscopy
1H NMR
1H NMR spectral data in deturated DMSO solution of the synthesized compounds are given in Table 2 The resonance of protons has been assigned on the basis of their integration and multiplicity pattern.The 1H NMR spectra of the Schiff bases in DMSO exhibits signals at 9.218, 9.50, 9.4, 9.22 and 9.36ppm for compounds 1, 2, 3, 4, and 5, attributed to CH=N- protons, respectively. The multisignals within the 6.89-8.1ppm range are assigned to the aromatic protons of both rings. The 1H NMR spectra of the Schiff bases synthesized from Salicylic acid revealed a signal at 10.39 and 10.26ppm due to the -CH=N- group on compounds 6 and 7 respectively. The OH moiety of Salicylic acid was observed at 9.04 and 8.97ppm. It should be noted that the phenolic protons have always given a singlet in off-set at high δ values, thus confirming its involvement in an intramolecular hydrogen bond with the neighboring nitrogen atom [55]. The free NH2 protons usually show a broad singlet peak in a region at 4-6ppm [56].This signal is absent in the observed spectra of Schiff bases which indicates the formation of the Schiff bases.
Table 2: 1H NMR data a-e of compounds with general formula R1N=CHR2
|
Compound no. |
Molecular Formula |
OH (s) |
CH (s) |
C6H4 (m) |
X (s) |
X′ (s) |
|
1 |
C14H9ClN2S |
– |
9.218 |
6.90-7.741 (8H) |
– |
– |
|
2 |
C14H9ClN2S |
– |
9.50 |
7.03-7.72 (8H) |
– |
– |
|
3 |
C14H10N2OS |
9.4 (1H) |
9.403 |
6.95-8.073 (8H) |
– |
– |
|
4 |
C14H10N2S |
– |
9.22 |
6.92-8.123 (9H) |
– |
– |
|
5 |
C15H12N2O2S |
9.58 (1H) |
9.36 |
6.953-7.784 (7H) |
3.435 (3H) |
– |
|
6 |
C14H10ClNO3 |
9.04 (1H) |
10.393 |
6.746-7.53 (7H) |
– |
10.85 (1H) |
|
7 |
C14H11NO4 |
8.97 (1H) |
10.261 |
6.92-7.871 (7H) |
8.97 (1H) |
10.72 (1H) |
|
8 |
C13H10ClNO |
9.573 (1H) |
8.616 |
6.791-7.916 (8H) |
– |
– |
|
9 |
C13H10ClNO |
9.549 (1H) |
8.608 |
6.826-7.920 (8H) |
– |
– |
|
10 |
C13H11NO2 |
9.700 (1H) |
8.888 |
6.848-7.596 (8H) |
9.700 (1H) |
– |
|
11 |
C14H12ClNO2 |
9.612 (1H) |
8.428 |
6.755-7.494 (9H) |
9.700 (1H) |
3.838 (1H) |
|
12 |
C13H11NO |
9.60 (1H) |
8.112 |
6.839-7.510 (9H) |
– |
– |
13C NMR
The 13C NMR spectra provide further support for the structural characterization of the Schiff bases. 13C NMR spectral data of compounds (1-12) have been listed in Table 3. The number of signals found corresponds with the presence of magnetically nonequivalent carbon atoms, which were assigned by comparison with literature values. The aromatic carbon present in the structures of Schiff bases were assigned by comparing the experimental chemical shifts with those calculated from the incremental method [57].The 13C-NMR spectral data of the Schiff bases are in accord with the proposed structures.
Table 3: 13C NMR data a-e of compounds with general formula R1N=CHR2
|
Compd. no. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
CH |
118.20 |
118.20 |
117.42 |
118.21 |
118.19 |
105.87 |
106.69 |
156.99 |
157.56 |
160.63 |
157.55 |
157.38 |
|
X |
– |
– |
– |
– |
55.63 |
167.35 |
172.11 |
– |
– |
– |
55.97 |
– |
|
X′ |
151.76 |
152.55 |
151.69 |
152.40 |
152.63 |
– |
– |
– |
– |
– |
– |
– |
| R1: C1 |
128.73 |
128.37 |
125.95 |
128.80 |
124.87 |
131.17 |
129.66 |
156.20 |
156.80 |
157.45 |
156.14 |
152.88 |
|
C2 |
125.80 |
125.89 |
125.74 |
125.72 |
125.79 |
121.24 |
119.93 |
116.19 |
116.20 |
116.43 |
115.77 |
116.33 |
|
C3 |
128.73 |
128.37 |
125.95 |
128.80 |
124.87 |
158.53 |
156.29 |
130.28 |
131.29 |
132.66 |
128.78 |
130.34 |
|
C4 |
131.09 |
131.07 |
131.07 |
128.96 |
131.04 |
121.09 |
119.78 |
123.10 |
122.97 |
119.42 |
110.53 |
123.19 |
|
C5 |
129.81 |
129.61 |
129.72 |
130.60 |
129.68 |
134.21 |
134.43 |
130.28 |
131.29 |
132.66 |
128.78 |
130.34 |
|
C6 |
130.96 |
130.97 |
131.07 |
128.96 |
131.09 |
136.53 |
136.87 |
116.19 |
116.20 |
116.43 |
115.77 |
116.33 |
| R2: C7 |
127.17 |
127.92 |
119.93 |
128.60 |
118.16 |
116.29 |
117.16 |
123.10 |
128.72 |
116.93 |
122.60 |
128.44 |
|
C8 |
129.14 |
133.49 |
155.64 |
127.20 |
127.76 |
132.86 |
155.15 |
129.32 |
136.92 |
157.45 |
123.97 |
128.44 |
|
C9 |
128.80 |
127.92 |
119.93 |
127.14 |
154.91 |
116.29 |
117.16 |
129.32 |
128.72 |
116.93 |
116.11 |
128.44 |
|
C10 |
134.71 |
130.44 |
131.60 |
126.98 |
148.93 |
129.87 |
131.95 |
135.78 |
129.19 |
132.95 |
143.63 |
128.44 |
|
C11 |
128.80 |
127.33 |
120.30 |
127.14 |
131.82 |
127.66 |
122.74 |
129.32 |
143.06 |
119.42 |
150.11 |
128.44 |
|
C12 |
129.14 |
130.44 |
131.60 |
127.20 |
127.76 |
129.87 |
131.95 |
129.32 |
129.19 |
132.95 |
115.77 |
128.44 |
Antibacterial activity
The results of the antibacterial screening of the Schiff bases at a concentration of 20mg/ml against all bacteria have been found. The inhibition zones were measured in mm and results are shown in Table 4. The results of antimicrobial screening, indicate that Schiff bases show significant activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis than Aspergillus niger and Chalara corda while compound 1, 2 were found to be more active against all tested bacterial strains because of the presence of chloro group in the aldehydic group which itself is active against microbes.
Table 4: Antibacterial activity dataa-c of compounds with general formula: R1N=CHR2 (in vitro)
|
Compound no. |
Zone of inhibition of sample (mm) in: |
||
|
Escherichia coli |
Bacillus subtilis |
Staphylococcus auereus |
|
|
1 |
34 |
21 |
30 |
|
2 |
42 |
31.5 |
49 |
|
3 |
39.5 |
20 |
36 |
|
4 |
22 |
16 |
30 |
|
5 |
35 |
22 |
32 |
|
6 |
23 |
31 |
– |
|
7 |
22 |
18 |
21 |
|
8 |
26 |
35 |
30 |
|
9 |
26 |
24 |
36 |
|
10 |
24 |
31 |
49 |
|
11 |
17 |
31 |
37 |
|
12 |
20 |
24 |
30 |
|
Std. drug (Amoxycillin) |
18 |
12 |
14 |
Antibacterial activity of these compounds show ascending order. When we increase concentration, area of inhibited growth also increased.
Antifungal activity
From the results obtained by the antifungal activity it is found that the benzthiazole Schiff bases are more active against all tested fungi then the salicylic acid Schiff bases. Compound 1, 2, 3, 4 and 5 are the most potent candidates against all type of tested fungi. The greater activity of these compounds is probably due to the presence of benzthiazole moiety. Compound 7 show good activity against all tested fungi as compared to standard drug. Compound 6 is significantly active against Aspergillus niger. The antifungal activity results are shown in Table 5.
Table 5: Antifungal activity dataa-d of compounds with general formula: R1N=CHR2 (in vitro)
|
Compound no. |
Zone of inhibition of sample (mm) in: |
|
|
Aspergillus niger |
Chalara corda |
|
|
1 |
47 |
37 |
|
2 |
45 |
33 |
|
3 |
41 |
32 |
|
4 |
40 |
32.5 |
|
5 |
42 |
34 |
|
6 |
22 |
– |
|
7 |
28 |
28 |
|
8 |
31 |
28 |
|
9 |
36 |
37 |
|
10 |
27 |
34 |
|
11 |
32 |
38 |
|
12 |
33 |
25 |
|
Std. drug (Ciprofloxacin) |
20 |
12 |
Conclusion
Schiff bases of 2-amino-Benzthiazole, 4-amino-Salicylic acid and 4-aminophenol were synthesized and characterized by analytical and spectral techniques. These compounds exhibited significant activity against all the tested microorganisms.
Acknowledgment
The work reported in this paper was carried out in Analytical Laboratory, Department of Chemistry, University of Malaya, Kuala Lumpur, Malaysia through UM Research Grant vide no. PS355/2009C. Thanks also to the Ministry of Higher Education Malaysia (MOHE) for financial support to Muhammad Aqeel Ashraf.
References
- Z. Cimerman, S. Miljanic and N. Galic, Croatica Chemica Acta, 2000, 73 (1), 81- 95.
- P. Singh, R. L. Goel and B. P. Singh, J. Indian Chem. Soc., 1975, 52, 958.
- B. F. Perry, A. E. Beezer, R. J. Miles, B. W. Smith, J. Miller and M. G. Nascimento, Microbois., 1988, 45, 181.
- A. Elmali, M. Kabak and Y. Elerman, J. Mol. Struct., 2000, 477, 151.
- P. R. Patel, B. T. Thaker and S. Zele, Indian J. Chem., 1999, 38 A, 563.
- M. Valcarcel and M. D. Laque de Castro, “Flow–Throgh Biochemical Sensors“, Elsevier, 1994, Amsterdam.
- U. Spichiger-Keller, “Chemical Sesors and Biosensors for Medical and Biological Applications”, Wiley-VCH, 1998, Weinheim.
- J. F. Lawrence and R. W. Frei, “Chemical Derivatization in Chromatography”, Elsevier, 1976, Amsterdam.
- S. Patai, Ed., “The Chemistry of the Carbon-Nitrogen Double Bond”, J. Wiley & Sons, 1970, London.
- E. Jungreis and S. Thabet S, “Analytical Applications of Schiff bases”, Marcell Dekker, 1969, New York.\
- Metzler C M, Cahill A and Metzler D E, J. Am. Chem. Soc., 1980, 102, 6075.
- G. O. Dudek and E. P. Dudek, Chem. Commun., 1965. 464.
- G. O. Dudek and E. P. Dudek, J. Am. Chem. Soc., 1966, 88, 2407.
- Z. Cimerman and Z. Stefanac Z, Polyhedron, 1985, 4, 1755.
- N. Galic, Z. Cimerman and V. Tomisic, Anal. Chim. Acta, 1997, 343, 135.
- P. Pfeiffer, E. Breith, E. Llibbe and T. Tsumaki, Justus Liebigs Ann. Chem., 503, 84 (1933).
- L. Hunter and J.A. Marriott, J. Chem. Soc, 2000 (1937).
- L. Sacconi, M. Ciampolini, F, Maggio and F.P. Cavasini, J. Am. Chem. Soc., 84, 3246 (1962).
- R.H. Holm and K. Swaminathan, Inorg. Chem., 1, 599 (1962).
- G.C. Perryand, D.A.Thornton, J. Inorg.Nue.Chem, 34, 3357 (1972).
- E.M. Hodnett, W.J. Dunn, J. Med. Chem., 13,768 (1970).
- E.M. Hodnett, P.D. Mooney, J. Med. Chem., 13, 786 (1970).
- G.H. Alt (Monsanto Co.), US. 4.226.615 (1980); Chem. Abstr., 94, (1981) 26155
- Y. Hamada, I. Takeuchi, Y. Ita, S. Matsui and T. Ita, Yakugaku Zasslzi, 101, 633 (1981); Chem. Abstr., 95, 181559 (1981).
- M. Ismail, Indian J. Pharm. Sei., 45, 121 (1986); Chem. Abstr., 107,175589 (1987).
- P.R. Palet, B.T. Thaker, and S. Zele, Indian, J. Chem., A38 (1999)563.
- K.Y. Lau, A. Mayr, K.K. Cheung, Inorg. Chem. Acta, 285, 223 (1999).
- A.S, Shawali, N.M.S. Harb and K.O. Badahdah, J. Heterocylic Chem., 22, 1397(1985).
- A. Abbaspour, A.R. Esmaeilbeig, A.A.,Jarrahpour, B.,Khajeh and R. Kia, Talanta, 58 397 (2002).
- R.K. Mahajan, I. Kaur, M. Kumar, Sens. Actuators, B-91, 26 (2003).
- M.R. Ganjali, M. Golmohammadi, M. Yousefi, P. Norouzi, M. Salavati-Niasari and M. Javanbakht, Anal. Sci, 19, 223 (2003).
- A.K. Jain, V.K. Gupta, P.A. Ganeshpure and J.R. Raisoni, Anal. Chim. Acta, 553, 177 (2005).
- T. Jeong, H.K. Lee, D.C. Jeong, S. Jeon, Talanta, 65, 543 (2005).
- V.K. Gupta, A.K. Singh, S. Mehtab and B. Gupta, Anal. Chem. Acta, 566, 5 (2006).
- M.M. Hernandes, M.L. Mckee, T.S. Keizer, B.C. Yeaswood and D.A. Atwood, J. Chem. Soc., Dalton Trans 410 (2002).
- G. H. Olie, and S. Olive, Springer, Berlin (1984).
- S. Li, S. Chen, H.Ma, R. Yu and D. Liu, Corros. Sci, 41, 1273 (1999).
- H. Ashassi-Sorkhabi, B.Shabani, B. Aligholipour and D.Seifzadeh , Appl. Surf. Sci., 252, 4039 (2006).
- Z.Quan, S.Chen and Y. Li, Corros. Sci., 43 (2001)1071.
- D.R. Williams, Chem. Rev., 72, 203 (1972).
- A. Campos, J.R. Anacona and M.M. Campos-Vallette, Mian group Metal chem., 22, 283 (1999).
- N. Sari, S. Arslan, E. Logoglu and I. Sakiyan, G.U.J. Sci, 16, 283 (2003).
- M. Verma, S.N. Pandeya, K N. Singh, J P. Stabler and Acta Pharm., 54, 49 (2004).
- P.G. Cozzi, Chem. Soc. Rev., 410 (2004).
- S. Chandra, J. Sangeetika, J. Indian Chem. Soc, 81, 203 (2004).
- A.M. Mahindra and J.M. Fisher, Rabinovitz., Nature (London), 303, 64 (1983).
- S.N. Pandeya, P. Yogecswari, D. Sriram, Chemotherapy, 45,192 (1999).
- W.J. Sawodny and M. Riederer, Angew. Chem. Int. Edn. Engi. 16, 859 (1977).
- A. Bottcher, T. Takeuchi, M.I. Simon, T.J. Meade and H.B. Gray, J. Inorg. Bio-Chem., 59, 221 (1995).
- G.L.P. Britovsek, V.V. Gibson, S. Mastroianni, D.C.H. Oakes, C. Redshaw, G.A. Solan, A.J.P. White, D.J. Williams, Eur. J. Inorg. Chem., 431, 2 (2001).
- B. Sun, J. Chen, J.Y. Hu, Lix., J. Chin. Chem. Soc, 12, 1043 (2001).
- D.M. Boghaei and S. Mohebi, , Tetrahederon, 58, 5357 (2002).
- S.Y. Liu, D.G. Nocera, , Tetrahedron Lett., 47, 1923 (2006).
- A. Budakoti, M. Abid and A. Azam, Eur. J.Med. Chem., 41, 63 (2006).
- G. Roman, M. Andree, Bulletin of the Chemists and Technologists of Macedonia, 20, 131 (2001).
- D.L. Pavia, G.M. Lampman, G.S. Kriz, Harcourt Brace College Publishers, America (1996).
- M. Danish, S. Ali, A. Badshah, M. Mazhar, H. Masood, A. Malik and G. Kehr, Inorg. Met.-Org. Chem., 27, 863 (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.









