Sensitized Photo-Oxidation of P-Aminobenzoic Acid by Fenton Reagent
Dharmendra Kumar
Department of Chemistry, M.S.J. Government College, Bharatpur - 321 001, India.
Sensitized photo-oxidation of p-aminobenzoic acid by Fenton reagent was studied. The effect of various reaction parameters such as substrate, H2O2, FeSO4, pH, polarity of solvent and catalyst variation was studied. The progress of the reaction was observed by TLC. Photoproduct was characterized by physical, chemical and spectral methods. A tentative mechanism has been proposed with overall reaction.
KEYWORDS:Sensitized photo-oxidation; p-aminobenzoic acid; Photo Fenton reagent.
Download this article as:| Copy the following to cite this article: Kumar D. Sensitized Photo-Oxidation of P-Aminobenzoic Acid by Fenton Reagent. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Kumar D. Sensitized Photo-Oxidation of P-Aminobenzoic Acid by Fenton Reagent. Available from: http://www.orientjchem.org/?p=24736 |
Introduction
Fenton and photo-Fenton processes are based on the generation of hydroxyl radicals that is considered as advanced oxidation processes (AOPs). Hydroxyl radicals are extraordinarily reactive and unstable species that attack most of the organic pollutants1,2.
These treatment processes are considered as very promising methods for the remediation of contaminated ground, surface and wastewaters containing non-biodegradable organic pollutants.
Fenton and photo-Fenton reagent was found to be very effective in treating various industrial wastewater including recalcitrant pollutants3, diurane and linuron herbicides4 a wide variety of dyes5,6 and landfill leachate7.
Material and Method
p-Aminobenzoic acid (Merck, Germany), ferrous sulphate (Merck, India), hydrogen peroxide 30% (Merck, India), sulphuric acid (Merck, India) and methanol (Rankem, India) were used to prepare all the solutions. Besides methanol, the rate of reaction was also studied in solvents like ethanol, acetone and ethyl acetate. All melting points were recorded on Toshniwal melting point apparatus. The phmeasurements were done with the help of Systronics-327 Griph (digital) ph meter. An Infra Red spectrum was scanned on SCHIMADZU FTIR-8400S spectrophotometer. Elemental analysis was carried out using Carlo-Erba-1106 automatic analyzer.
p-Aminobenzoic acid (0.25 gm) was dissolved in methanol in a round bottom flask, solution of ferrous sulphate (2.0 ml, 0.1M), hydrogen peroxide (0.35 ml, 30%) and sulphuric acid (0.5N) were added for maintaining ph. Total volume of the reaction mixture was made 100 ml by adding methanol. All the chemicals used in the investigation were purified according to the recommended methods. The concentration of various ingredients in the reaction mixture were p-Aminobenzoic acid 18.3 X 10-3 M, Feso4 2.0 X 10-3 M, H2O2 31.5 X 10-3 M and the ph of the solution was found to be 2.4.
The reaction mixture was irradiated with light source (Tungsten lamps, 2 x 200W, Philips) at a distance of 30 cm from the reaction vessel. A water filter (15 cm thick) was placed between light source and the reaction vessel to cut off thermal radiations.
The progress of the reaction was observed with the help of tlc, at every 2 h interval and the product was identified by its usual tests. In initial stages of reaction, only a single spot corresponding to parent compound was observed when the tlc plate was placed in iodine chamber. After 4 h, two spots corresponding to parent compound and photoproduct were observed. The reaction was allowed for completion (7 h).
The rate of the oxidation depends on various parameters like substrate, H2O2, Feso4, ph, polarity of solvent and the catalyst variation. The results of these variations are as follows:
Effect of substrate concentration
The effect of concentration of substrate on photo catalytic reaction was studied using variable amount of substrate, those were 10.9 X 10-3 M, 14.6 X 10-3 M, 18.3 X 10-3 M, 22.0 X 10-3 M and 25.7 X 10-3 M . The % yield of photoproduct was 20.5, 22.6, 30.4, 29.8 and 28.7. It has been observed that as the concentration of substrate increases, the yield of photoproduct was found to increase, up to an optimum level. On further increase in concentration of substrate, yield of photo product was decreased. It may be due to the fact that as the concentration of the substrate was increased, only a fraction of the light intensity will reach the catalyst surface and thus; a decrease in the photocatalytic oxidation of substrate was observed.
Effect of hydrogen peroxide concentration
The effect of concentration of hydrogen peroxide on the yield of photoproduct was investigated using different concentration of H2O2, those were 22.5 X 10-3 M, 27.0 X 10-3 M, 31.5 X 10-3 M, 36.0 X 10-3 M and 40.5 X 10-3 M . The % yield of photoproduct was 19.6, 21.8, 30.4, 30.1 and 29.5.
As the concentration of hydrogen peroxide was increased, the yield of photoproduct also increases. However, above a certain H2O2 concentration, the reaction rate levels off and is negatively affected. This may be due to auto-decomposition of H2O2 to oxygen and water and recombination of OH˙ radical.
Effect of ferrous ion concentration
The effect of Fe2+ concentrations was studied using various concentrations of Fe2+ ions, those were 1.0 X 10-3 M, 1.5 X 10-3 M, 2.0 X 10-3 M, 2.5 X 10-3 M and 3.0 X 10-3 M. The % yield of photoproduct was 18.4, 22.0, 30.4, 28.6 and 27.4.
From above observations it can be concluded that as the concentration of Fe2+ ions is increased the rate of reaction also increases, up to a certain limit. But after reaching on optimum level the efficiency decrease. This may be due to the increase of a brown turbidity that hinders the absorption of the light required for the photo-Fenton process or by the recombination of OH˙ radical. In this case, Fe2+ reacts with OH˙ radical as scavenger.
Effect of ph variation
Keeping all the other conditions identical, the effect of ph on the photo oxidation was studied, those were 1.7, 2.0, 2.4, 2.8, 3.1. The % yield of photoproduct was 18.0, 22.4, 30.4, 24.1 and 20.4.
These observations are showing that the rate of reaction increases up to a certain limit (2.4). With further rise in pH, the yield of the photoproduct is decreased. The drop in efficiency on the basic side is attributed to the transition of iron from a hydrated ferrous ion to a colloidal ferric species. In this form, iron catalytically decomposes the H2O2 into oxygen and water, without forming hydroxyl radical.
Effect of polarity of solvent
The effect of polarity of solvent was observed using a wide range of solvents with different polarity; those were Ethyl acetate, Acetone, Ethanol and Methanol. The % yield of photoproduct was 15.2, 19.4, 23.6 and 30.4. It was observed that the rate of photo oxidation increased with the increase in the polarity of the solvent.
Effect of catalyst variation
Keeping all the other conditions identical, the effect of catalyst variation on the photo oxidation was studied, those were H2O2 , H2O2+Fe2+ , H2O2 + UO22+ and H2O2 + UO22+ +Fe2+. The % yield of photoproduct was 21.2, 30.4, 33.1 and 34.2.
It was observed that when Fe2+ ions of Fenton reagent replaced by uranyl ions [UO22+], the percentage yield of photoproduct was increased. It may be due to the formation of more hydroxyl free radical which oxidizes the available organic matter.
Results and Discussion
After the completion of photo catalytic reaction, the photoproduct was characterized by its usual chemical tests 8,9.
Nitrogen was found to be present.
Black precipitates with Milliken Barker test confirm the presence of –NO2 group.
The photoproduct p-nitrobenzoic acid was separated as its amide derivative (recrystallized, M.P. 198 0C)
The control experiments were performed. The reaction was carried out in the presence of (i) Oxygen and light (no photo catalyst was added), (ii) Oxygen and photo catalyst (no exposure to light) and (iii) Light and photo catalyst (no oxygen was purged).
It was observed that no photoproduct had formed in the first cases and the yield was very low in second and third case. So it is concluded that both light and photo catalyst are necessary for the photo reaction and oxygen increases the rate of reaction. Involvement of free radicals has been confirmed by adding acryl amide in the reaction mixture where a resinous mass is obtained.
The I.R. spectrum shows the peak at 1530 cm– 1 and 1350 cm-1 which confirms the presence of No2 group. There is no absorption in region 3500-3300 cm-1 (N-H stretching) and 1640-1560 cm-1 and 800 cm-1 (N-H bending) confirms the absence of NH2 group in the photoproduct.10,11.
On the basis of the above, the following mechanism (Fig.1) has been proposed for the photo catalytic reaction of p-aminobenzoic acid with Fenton reagent. The generally accepted mechanism for the Fenton process identifies the hydroxyl radical (•OH) and hydroperoxy radical (HO2·) as the active oxidizing intermediate in the system. According to this mechanism, the combination of ferrous iron and hydrogen peroxide induces a series of chain reactions initiated by the degradation of peroxide to the Fe3+,hydroxyl radical and the hydroxide ion (reaction 1).
Fe2+ + H2O2→Fe3+ + OH ˉ + OH· (1)
Hydrogen peroxide decomposes catalytically by Fe (III) and generates hydroperoxy radicals and Fe2+ (reaction 2 & 3).
Fe3+ + H2O2→H+ + [Fe-HO2]2+ (2)
[Fe-HO2]2+→HO2· + Fe2+ (3)
The hydroxyl radical and hydroperoxy radical reacts with substrate to give the product respectively by reaction 4 and 5. The OH·radical formed from the photolysis brings about a radical chain mechanism forming HO2·, O2, .OH· etc. which are used for the oxidation of substrate12,13.
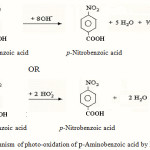 |
Figure 1: Mechanism of photo-oxidation of p-Aminobenzoic acid by Fenton reagent |
Acknowledgement
The authors are thankful to U.G.C. Regional Office, Bhopal for providing Minor Research Project.
References
- Glage, W. H. and Kang, J. W. : Ind. Eng. Chem.Res., 28, (1989) 1580.
- Bigda, R. J. : Chem. Eng. Progr., 91,(1995) 62.
- Oller, I., Malato, S., Sanchez-Perez, J. A., Maldonado, M. I., Gernjak, W. and Perez-Estrada, L. A. : Water Sci. Tecnol., 55 (12), (2007) 229.
- Farre, M. J., Garcia-Montano, J., Ruiz, N., Munoz, I., Domenech and Peral, J. : Environ. Technol., 28 (7), (2007) 819.
- Monteagudo, J. M., Durán, A. and López-Almodóvar, C. : Applied Catalysis B: Environmental, 83 (1-2), (2008) 46.
- Orozco, S. L., Bandala, E. R., Arancibia-Bulnes, C. A., Serrano, B., Suárez-Parra, R. and Hernández-Pérez, I. : J. Photochem. Photobiol. A: Chem., 198 (2-3), (2008) 144.
- Deng, Y. : J. Hazard Meter., 146 (1-2), (2007) 334.
- Visnoi, N. K. : Advanced Practical Organic Chemistry, 2nd Rev. Ed. Vikas Publishing Pvt. Ltd., New Delhi, (2000).
- Vogel, A. L. : Text book of Practical Organic Chemistry, 4th Ed. ELBS Publishing, London, (1978).
- Silverstein, R. M. and Webster, F. X. : Spectrometric Identification of Organic Compounds, John Willey and Sons, Inc., 6th Ed. New York, (1998).
- Williams, D. H. and Fleming, I. : Spectroscopic Methods in Organic Chemistry, 4th Ed. Tata Mc Graw Hill, New Delhi, (1990).
- Fenton, H. J. H. : J. Chem. Soc., 65, (1894) 899.
- Urey, H. C., Dawsey, L. H. and Rise, F. O. : Am. J. Chem. Soc., 51, (1929) 137.

This work is licensed under a Creative Commons Attribution 4.0 International License.









