Radiation-Induced Graft Copolymerization of Acrylonitrile Onto Kappa-Carrageenan
Hossein Hosseinzadeh
Department of Chemistry, Payame Noor University, 19395-4697, Tehran Iran.
The monomer, acrylonitrile, was graft copolymerized onto kappa-carrageenan (kC) using γ-rays as initiator. The reactions were carried out in a homogenous aqueous medium. After removal of the homopolymer, the graft copolymer was characterized by FTIR spectroscopy. A plausible mechanism of grafting has also been suggested. The effect of various factors affecting on grafting, i.e. dose of δ-rays and concentration of the monomer and polysaccharide as well as the reaction temperature were studied by conventional methods to achieve the optimum grafting parameters. The graft copolymerization reactions were kinetically investigated using semi-empirical expressions and a suitable rate expression has been derived.
KEYWORDS:Kappa-carrageenan; Acrylonitrile; Graft copolymerization; γ-irradiation
Download this article as:| Copy the following to cite this article: Hosseinzadeh H. Radiation-Induced Graft Copolymerization of Acrylonitrile Onto Kappa-Carrageenan. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Hosseinzadeh H. Radiation-Induced Graft Copolymerization of Acrylonitrile Onto Kappa-Carrageenan. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24855 |
Introduction
Graft copolymerization is an attractive means for modifying base polymers because grafting frequently results in the superposition of properties relating to the backbone and pendant chains. Considerable interest has been focused on chemical modification by free radical graft copolymerization of hydrophilic and hydrophobic vinyl monomers biopolymers such as polysaccharides [1-3]. These biodegradable and low cost graft copolymers, with new properties, can be used in many applications such as textiles, paper industry, agriculture, medical treatment and also in petroleum industry as flocculants and thickening agents [4, 5].
Graft copolymers are prepared by first generating free radicals on the polysaccharide backbone and then allowing these radicals to serve as macroinitiators for the vinyl monomers. Graft copolymerization can be carried out with different initiator systems. Among them, potassium persulfate, ammonium persulfate, benzoyl peroxide, azo bisisobutyronitrile, and ceric ammonium nitrate are widely used for the synthesis of graft copolymers [6, 7].
Radiation grafting technology is well established and accepted by industry. Radiation polymerization, radiation crosslinking and controlled degradation of polymers comprise most of commercial applications of radiation technology [8].
The chosen polysaccharide for modification, i.e. kappa-carrageenan, kC, is the most well-known and most important type of carrageenan family. Carrageenan is a collective term for linear sulfated polysaccharides that are obtained commercially by alkaline extraction of certain species of red seaweeds [9]. Schematic diagram of the idealized structure of the repeat units for the kC, is shown in Figure 1.
Of the monomers grafted, acrylonitrile has been the most frequently used one, mainly due to its highest grafting efficiency [10, 11], improving the thermal resistance of the graft copolymer [12] and also the subsequent alkaline hydrolysis of the grafting product to obtain water absorbents [10, 13].
The present report describes graft copolymerization of acrylonitrile onto kappa-carrageenan backbone, initiated by γ-rays.
Experimental
Materials
The polysaccharide, kappa-carrageenan (high MW, purified, from Condinson Co., Denmark) was used without further purification. Acrylonitrile (AN, from Merck) was used after vacuum distillation for removing inhibitor.
Grafting procedure
Graft copolymerization of acrylonitrile onto kappa-carrageenan was carried out with γ-rays initiator. In a 100 mL flask, certain amount of kappa-carrageenan (0.5-3.0 g) was dissolved in 50 mL of degassed distilled water. The flask was placed in a water bath with desired temperature (60 oC). A given amount of monomer, AN (1.5-5.0 g), was added to the flask and the mixture was stirred for 15 min. The cold mixture was removed into a 250 mL aluminium tube. The inner wall of aluminium tube was covered with aluminium foil. The tube was closed tightly with the foil and paraffin film. The tube was then irradiated under γ-rays according to the desired total doses.
Homopolymer extraction
The graft copolymer was freed from polyacrylonitrile (PAN) homopolymer, by pouring 0.50 g of the product in 50 mL of dimethyl formamide solution. The mixture was stirred gently at room temperature for 24 h. After complete removal of the homopolymer, the copolymer was filtered, washed with ethanol and dried in oven at 50˚C to reach a constant weight.
Evaluation of grafting parameters
The grafting parameters used to characterize the nature of the copolymer are defined with the weight basis expressions as reported by Fanta [4]. The percentage of grafting ratio (Gr%) stands for the weight percent of the graft copolymer synthetic part (PAN grafted) formed from initial sodium hyaluronate used.
![]()
The percentage of grafting efficiency (Ge%) stands for the grafted PAA formed from initial monomer charged.
![]()
The percentage of Add-on (Ad%) is the weight percent of the grafted PAN of the graft copolymer.
![]()
The percentage of homopolymer (%Hp) denotes the weight percent of the homopolymer formed from initial monomer charged.
Homopolymer(%Hp)=100-%Ge
Instrumental analysis
FTIR spectra of samples in the form of KBr pellets were recorded using an ABB Bomem MB-100 FTIR spectrophotometer. Irradiation was carried out using γ-rays from 60Co source, in a Gammacell-220 (Nordion, Canada) with a dose rate of 1.6 kGy/h, in air and at room temperature. The dose rate was determined by the convention al Fricke dosimeter.
Results and Discussion
Graft copolymerization mechanism
The mechanism of grafting acrylonitrile (AN) onto kappa-carrageenan (kC) using γ-rays as an initiator is shown in the Scheme 1. It should be mentioned that during the irradiation of AN, kC and water ternary mixture, most of the energy is absorbed by water and only a very small fraction by other components. Thus, the initiation occurs mainly by an indirect effect. Hydroxyl radicals, formed during irradiation, add to one side of the AN double bond and leads to the formation of an unpaired spin on the other side of the vinyl bond. In this way, homo polymerization of AN is initiated. Attack of OH radicals on carrageenan would lead almost solely to the break age of C–H bonds. This fact is very well known from radiation chemistry of alcohols and carbohydrates in aqueous solution. A much more probable pathway is the addition of a AN molecule (not a radical) to the carrageenan-based radical, followed by polymerization leading to the growth of a branched chain.
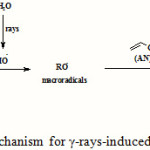 |
Scheme 1: A brief proposed mechanism for γ-rays-induced grafting of poly(AN) onto kC. |
FTIR spectroscopy
Infrared spectroscopy is the best tool to confirm the grafting reaction. The IR spectra of pure kC and the graft copolymer, kC-g-PAN, was shown in Figure 1. In the IR spectrum of kC, the peaks observed at 845, 912, 1026, and 1221 cm-1 could be related to D-galactose-4-sulfate, 3,6-anhydro-Dgalactose, glycosidic linkage, and ester sulfate stretching of kC, respectively (Figure 1a). The broad band at 3200–3400 cm-1 is due to stretching of -OH groups of kC. An additional sharp characteristic peak in the graft copolymer at 2246 cm-1 (Figure 1b) which is attributed to –C≡N stretching absorption can be used for confirming the grafting of PAN onto the substrate, kC.
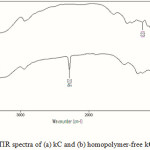 |
Figure 1: FTIR spectra of (a) kC and (b) homopolymer-free kC-g-PAN. |
Reaction rate
The rates of polymerization (Rp) and graft copolymerization (Rg) may be evaluated as measures of the rate of monomer disappearance by using the following equations [13]:
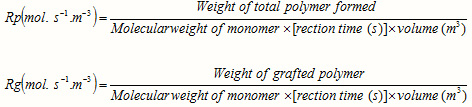
The calculation of Rp values may be of significant importance in confirming a proposed reaction mechanism and kinetics. Therefore, we investigated the relation between rate of graft copolymerization and concentration of AN and kC. Figures 2 and 3 show that the plots of Rp versus the monomer concentration, [AN] and half-order of the polysaccharide concentration, [kC]1/2 are linear. This is in agreement with a modified kinetic scheme already explored for CAN-initiated acrylonitrile grafting onto carboxymethyl cellulose [14]. The statement of rate of polymerization according to the scheme is as follows:
Rp = kp (K kd / kt )1/2 [ kC ]1/2 [AN]
The coefficient K is the equilibrium constant; kp, kd, and kt are the rate constants for propagation, dissociation, and termination reactions, respectively. Therefore, we preliminarily conclude that the radiation-initiated grafting of AN onto kC is also fitted with this kind of rate statement.
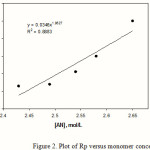 |
Figure 2: Plot of Rp versus monomer concentration. |
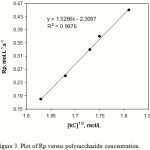 |
Figure 3: Plot of Rp versus polysaccharide concentration. |
Effect of polysaccharide concentration
Figure 4 shows the effect of kC concentration on the grafting parameters. With increasing the kC amount, more reactive grafting sites are formed which are favourable for grafting. This can account for initial increment in grafting parameters up to 8.0 wt% of kC value. Beyond this amount, the grafting values were diminished. This may be ascribed to the increase in viscosity that restricts the movement of the monomer molecules in a relatively small volume of the reaction mixture of 50 mL, and the termination reaction between macroradical-macroradical and macroradical-primary radicals as well.
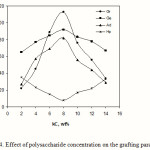 |
Figure 4: Effect of polysaccharide concentration on the grafting parameters. |
Effect of δ-rays dose
Graft copolymerization was studied at various doses of δ-rays by keeping other reaction conditions constant. As shown in Figure 5, the %Ge and %Gr increase with increasing in the doses of δ-rays and reach at a maximum value. Further increase of doses of δ-rays beyond 30 kGy disfavoured the grafting parameters. A relatively high dose of δ-rays may cause a reduction of %Ge and %Gr due to increase in the number of kC free radicals terminated prior to AN addition. Furthermore, homopolymer formation at higher doses of δ-rays which compete with the grafting reaction for available monomer could lead to decrease in the %Ge and %Gr.
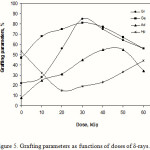 |
Figure 5: Grafting parameters as functions of doses of δ-rays. |
Effect of monomer concentration
The effect of AN concentration on the grafting parameters is presented in Figure 6. In the initial stages, though both %Ge and %Gr rise with increase in AN concentration, but beyond certain concentration of monomer, 0.6 mol/L, the grafting parameters decrease. The initial increase in grafting parameters could be associated with the greater availability of monomer molecules in the vicinity of kC macroradicals. The decrease of %Gr and %Ge with further increase in the AN concentration may be explained as follows: (a) preferential homopolymerization over graft copolymerization, (b) increasing the viscosity of reaction medium, which hinders the movement of free radicals, and (c) increase in the chance of chain transfer to monomer molecules.
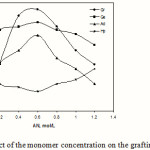 |
Figure 6: Effect of the monomer concentration on the grafting parameters. |
Effect of reaction temperature
Figure 7 shows the influence of reaction temperature on grafting. An increase in temperature upto 70 oC increases the grafting parameters. This behaviour may be due to the higher rate of diffusion of acrylonitrile molecules to polysaccharide macroradicals. Moreover, higher temperatures increase the solubility of the reactants. The subsequent lower grafting can mainly be explained on the basis of increasing rate of termination and chain transfer reactions.
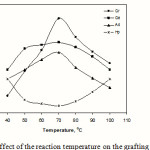 |
Figure 7: Effect of the reaction temperature on the grafting parameters. |
Conclusion
The polysaccharide, kappa-carrageenan was graft copolymerized with synthetic monomer, acrylonitrile, using δ-rays as efficient free radical initiators. In order to prove that monomer molecules were grafted, FTIR spectroscopy was used. The synthetic conditions were systematically optimized through studying the influential factors including temperature, doses of δ-rays as well as concentration of the monomers and polysaccharide. The effect of the individual factors was investigated by calculating the grafting parameters. The relation between the rate of polymerization (Rp) and the concentrations of reactants was also investigated. Overall, the grafted polysaccharide may be a candidate for manufacture of moulded plastics, ion exchange resins, and plastic films and in cosmetics. On the other hand, since non-biodegradable plastic waste is known as an ecological threat, such natural polymer-based plastics in fact, are the need of time. Hence, improving the thermal stability of the polysaccharides would make them better suited for, for instance, moulded articles.
References
- Jenkins D. W., Hudson S. M. Chem. Rev. 101, 3245 (2001).
- Joshi J. M., Kumar S. V. Polymer, 47, 2198 (2006).
- Sun T., Xu P., Liu Q., Xue J., Xie W. Europ. Polym. J. 39, 189 (2003).
- Fanta G. F. Block and Graft Copolymerization, R. J. Cerasa (Ed.), Wiley, London (1973).
- Hebeish A., Guthrie J. T. The Chemistry and Technology of Cellulosic Copolymers, Springer-Verlag, Berlin (1981).
- Tripathy T., Singh R. P. Europ. Polym. J. 36, 1471 (2000).
- Pourjavadi A., Zohuriaan-Mehr M. J., Pooraghaberar A., Hosseinzadeh H. J. Polym. Mater., 21, 351 (2004).
- Hegazy E. A., Abdel-Rehim H.A., Kamal H., Kandeel K.A., Nuc. Inst. Meth. Phys. Res. B, 185, 235 (2001).
- Kirk R.E., Othmer D. F. Encyclopedia of Chemical Technology, 4th ed, vol. 4. New York: John Wiley & Sons (1992).
- Fanta G.F. Doane W.M., Grafted Starches. In Modified Starches: Properties and Uses; Wurzburg, O.B., Ed.; CRC Press: Boca Raton, Florida, p. 149 (1986).
- Sandle N.K., Verma O.P.S., Varma I.K., Thermochim. Acta, 115, 189 (1987).
- Athawale V.D., Rathi S.C., J. Macromol. Sci.-Rev. Macromol. Chem. Phys. C39, 445 (1999).
- Pourjavadi A., Zohuriaan-Mehr M. J. Starch/Starke 54, 140 (2004).
- Zohuriaan-Mehr M. J. Pourjavadi, A. Sadeghi M. Iran. Polym. J. 14, 131 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









