Preparation and Characterization of Metronidazole Loaded Carboxymethyl Cellulose-Base Superabsorbent for Drug Delivery Application
Mohammad Sadeghi1* and Behrouz Heidari2
¹Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak (Iran). ²Department of Chemical Engineering, Engineer Faculty, Islamic Azad University, Arak Branch, Arak, (Iran).
Article Received on :
Article Accepted on :
Article Published : 01 Jun 2011
The present work focused on the design of drug delivery system (DDS) based on a pH-,saltand temperature sensitive superabsorbent hydrogel.The novel biopolymer-based superabsorbent hydrogels were prepared by grafting crosslinked poly(AA-co-BuMC) chains onto CMC backbones through a free radical polymerization method. the hydrogel,s structure was confirmed using FTIR spectroscopy. Results from scanning electron microscopy (SEM) observation also showed a porous structure with smooth surface morphology of the hydrogel. Due to the reversible swelling behavior of the hydrogels, the synthesized networks can sense the environmental pH,ionic strength and temperature change, respectively, and achieve an oscillatory release pattern. Swelling profiles obtained clearly indicated that these hydrogels swell slightly in a simulated gastric fluid (SGF) and strongly in a simulated intestinal fluid (SIF). Using drug metronidazole(MZ) as a model molecule, the in vitro controlled drugrelease behaviors of these hydrogels were investigated. The release proûles of MZ from the hydrogel were determined by UV–Vis absorption measurement at λmax 278 nm. The metronidazole drug, was successfully loaded into the hydrogels and in vitro release studies were performed in SGF for the initial 70 min, followed by SIF until complete dissolution. The release of metronidazole was continued up to 150 min. The release mechanism of the hydrogels was also studied using the Ritger-Peppas model.
KEYWORDS:CMC; hydrogel; swelling; acrylic acid; butylmethacrylate; drug delivery
Download this article as:| Copy the following to cite this article: Sadeghi M, Heidari B. Preparation and Characterization of Metronidazole Loaded Carboxymethyl Cellulose-Base Superabsorbent for Drug Delivery Application. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Sadeghi M, Heidari B. Preparation and Characterization of Metronidazole Loaded Carboxymethyl Cellulose-Base Superabsorbent for Drug Delivery Application. Available from: http://www.orientjchem.org/?p=11682 |
Introduction
Hydrogels have been of interest to biomaterial scientists for many years because of their hydrophilic character and potential to be biocompatible (1-8). These networks are special soft and pliable polymeric materials that can be absorb large quantities of water, saline or physiological solutions while the absorbed solutions are not removable even under pressure(2).
To date, many types of hydrogels as drug carriers have been widely investigated. Some interesting drug delivery systems based on hydrogels have thus been proposed (9-12). Among hydrogels, however, considerable research attention has been focused on so-called “smart” hydrogels which can transfer their volume in response to environmental stimuli, and this result in environmental stimuli modifying drug release.
Indeed,the use of new natural polymers as drug carriers has received considerable attention in the last few years. One of the goals of such systems is to prolong the residence time of a drug carrier in the gastrointestinal (GI) tract. The bioadhesive bond can be of a covalent, electrostatic, hydrophobic, or hydrogen bond nature(3).
Ionic polymers are reported to be promising for bioadhesive medical applications, and an increased charge density will also give better adhesion, suggesting that the electrostatic interactions are of great importance.
Metronidazole (MZ) is an active adjunct in the treatment of H. pylori (15) with the commonly reported side effects including anorexia, nausea, vomiting, and epigastric pain. Metallic taste, mouth dryness, probably caused by the presence of high concentrations of the drug in the saliva, and furring of the tongue are also reported (16). This study focuses on the formulation of MZ–CMC and synthesis,characterization of a smart superabsorbing hydrogel from CMC-g-(PAA-co-PBuMC). Such dosage form for MZ would be beneficial in delivering higher concentrations of the antibacterial agent in the GI tract so as to facilitate investigation of the influence of CMC on drug release properties. In this work, we have sought to validate the concept of diffusion controlled release, since the diffusion of drug molecules within a hydrogel matrix is hindered by the insoluble network in which drug molecules have to travel through tortuous pathways to exit the matrix. Different The slow drug release suggests a controlled prolonged pathway for the drug to travel through the polymer network(14).
Experimental
Materials
The polysaccharide used in this work was carboxymethylcellulose (CMC) with D.S. 0.52. Ceric ammonium nitrate (CAN) was purchased from Merck and was used without purification. It was as freshly prepared 0.1 M solution in 1N HNO3. Acrylic acid,(AA, Merck) and Butylmethacrylate (BuMC,Fluka, Buchs, Switzerland) were used after distillation for removing inhibitor. The antibiotic metronidazole with characterization (Mw= 171.2 g/mol, Half Life = 6–12hour) was purchased from Sigma Aldrich. The chemical structure of metronidazole loaded is shown in Figure 1. All other chemicals were of analytical grade.
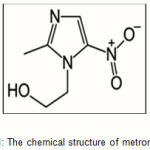 |
Figure 1: The chemical structure of metronidazole Click here to View figure |
Drug loading on hydrogels
The hydrogels were impregnated with drugs using a contact adsorption technique. The swollen hydrogel sample was dried in vacuum overnight until its weight remained unchanged. The vacuum dried powdered samples (1±0.0001 g), with average particle sizes between 40 and 60 mesh (250–350 ), were accurately weighted and immersed in the aqueous solution of drug (0.6 g dissolved in 50 mL distilled water) at 0oC for 24h to reach the equilibrated state. The swollen hydrogels loaded with drug were placed in a vacuum oven and dried under vacuum at 45 oC.
Standard absorbance curve
The standard calibration curve of the absorbance as a function of drug concentration was studied at 278 nm on the UV spectrophotometer.
Determination of loading efficiency
The amount of drug content entrapped in the hydrogels was determined by an indirect method. After the gel preparation, the washings were collected, filtered with a 0.45 Millipore filter and tested at λmax 278 nm using UV/VIS spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan).
The drug entrapped exhibited the same λmax as the free drug. This clearly indicates that the drugs entrapped have not undergone any possible chemical reaction during the matrix formation. The difference between the amount of drug initially employed and the drug content in the washings is taken as an indication of the amount of drug entrapped (12):
![]()
In vitro drug release
The samples (0.1±0.0001 g) were immersed into 50 mL of the release medium (simulated gastric and intestinal fluids, SGF and SIF) with different pH values (pH 1.6 or 7.4) at 37oC with agitation. At given time intervals, 1 mL of the release medium was removed, using a syringe attached with a 0.45 Millipore filter and after suitable dilution, the concentration of released drug was measured by UV spectrophotometer at 278 nm.
Swelling and Deswelling Measurements
An accurately weighed sample (0.20 g) of the powdered superabsorbent with average particle sizes between 40-60 mesh (250–350 μm) was immersed in distilled water (200 mL) or desired salt solution (100 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capacity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method (14) and using the following formula:
![]()
The deswelling water ratio of each sample was evaluated from the following equation:
![]()
where Wt0 and Wt are the initial weight of the fully swollen sample and the weight of sample at the deswelling time, t, respectively.
Absorbency at Various pHs
Individual solutions with acidic and basic pHs were prepared by dilution of NaOH (pH 10.0) and HCl (pH 1.0) solutions to achieve pH≥6.0 and pH<6.0, respectively. The pH values were precisely checked by a pH-meter (Metrohm/620, accuracy ±0.1). Then, 0.5 g (± 0.001 g) of the dried hydrogel was used for the swelling measurements according to Eq. 1. To study the pH-reversibility of the hydrogels, solutions with pH 1.6 and 7.4 were used. Swelling capacity of the hydrogels at each pH was measured according to Eq. 1 at consecutive time intervals (30 min)(18).
Results and Discussion
Synthesis and Spectral Characterization
Scheme 1 shows a simple structural proposal of the graft copolymerization of Acrylic acid and Buthylmethacrylate monomers on the CMC backbones and crosslinking of the graft copolymer. In the first step, the thermally dissociating initiator, i.e. CAN, is decomposed under heating (65oC) and then pair redox ce3+-ce4+ disconnect C-C bond from the CMC backbones to form corresponding macroinitiators(19-22). These macroradicals initiate grafting of AA and BuMC onto CMC backbones leading to a graft copolymer. Crosslinking reaction also occurred in the presence of the crosslinker, i.e. MBA(scheme 1).
FTIR spectroscopy was used for identification of the hydrogel. Figure 2 shows the IR spectra of the CMC and the resulted hydrogel. The band observed at 1636 cm-1 and can 1428 cm-1 be attributed to C=O stretching and bending in carboxylate(COO –) functional groups of substrate backbone (Figure 2a). The broad band at 2500-3500 cm-1 is due to stretching of –OH groups of the CMC. In the spectra of the hydrogel the characteristic band at 1716 cm-1 was attributed to C=O esther (BuMC monomer) stretching.
To obtain additional evidence of grafting, a similar polymerization was conducted in the absence of the crosslinker. After extracting the homopolymers, PAA or PBuMC and unreacted monomers using a cellophane membrane dialysis bag (D9402, Sigma–Aldrich), an appreciable amount of grafted CMC (91%) was observed. The graft copolymer spectrum was very similar to Figure 2b. Also according to preliminary measurements, the sol (soluble) content of the hydrogel networks was as little as 1.9 %. This fact practically proves that all AA and BuMC are involved in the polymer network. So, the monomers percent in the network will be very similar to that of the initial feed of reaction(24).
One of the most important properties that must be considered is hydrogel microstructure morphologies. Figure 3 shows the scanning electron microscope images of CMC and the hydrogel respectively. This picture verifies that the synthesized polymer in this work have a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the graft copolymers(24).
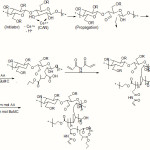 |
Scheme 1: A brief proposed mechanism for ceric-induced grafting of Acrylic acid and Buthylmethacrylate monomers onto CMC. Click here to View Scheme |
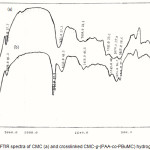 |
Figure 2: FTIR spectra of CMC (a) and crosslinked CMC-g-(PAA-co-PBuMC) hydrogel (b). |
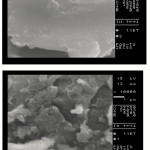 |
Figure 3: SEM photograph of the hydrogel. Surfaces were taken at a magnification of 10000, and the scale bar is 1 μm. Click here to View figure |
Salt-sensitivity behavior of CMC-g-poly(AA-co-BuMC) hydrogel
Swelling capacity in salt solutions is of prime significance in many practical applications such as personal hygiene products and water release systems in agriculture. The swelling ability of “anionic” hydrogels in various salt solutions is appreciably decreased compared to the swelling values in distilled water. This well-known undesired swelling-loss is often attributed to a “charge screening effect” of the additional cations which causing a non-perfect anion–anion electrostatic repulsion (17). Also, in salt solution the osmotic pressure resulting from the difference in the mobile ion concentration between gel and the aqueous phases is decreased and consequently the absorbency amounts are diminished. In addition, in the case of salt solutions with multivalent cations, “ionic crosslinking” at surface of hydrogel particles causing an appreciably decrease in swelling capacity.
Since the CMC-based hydrogels are comprised poly(NaAA) chains with carboxylate groups that can interact with cations, they exhibit various swelling capacity in different salt solutions with same concentrations. In the presence of the bivalent calcium ions, the crosslinking density increases because of a double interaction of Ca2+ with carboxylate groups leading to “ionic crosslinking”. The swelling–deswelling cycle of the hydrogel in sodium and calcium salts are shown in Figure 4. In sodium solution, swelling of the hydrogel is increased with time. When this hydrogel is immersed in calcium chloride solution, it deswells to a collapsed form. When the shrinked hydrogel is immersed in sodium chloride solution again, the calcium ions are replaced by sodium ions. This ion exchange disrupts the ionic crosslinks leading to swelling enhancement. As a result, when hydrogel is treated alternatively with NaCl and CaCl2 solutions with equal molarity, the swelling reversibility of hydrogel is observed(24).
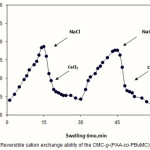 |
Figure 4: Reversible cation exchange ability of the CMC-g-(PAA-co-PBuMC)hydrogel. Click here to View figure |
pH-responsiveness behavior of CMC-g-poly(AA-co-BuMC) hydrogel
Ionic superabsorbent hydrogels exhibit swelling changes at a wide range of pHs. Therefore, in this series of experiments, we investigated the reversible and reproducible swelling-deswelling cycles behavior of The CMC-g-(PAA-co-PBuMC) in solutions with pH 1.6 and 7.4 (Figure 5). At pH 7.4, the hydrogel swells up to 75 g/g due to anion-anion repulsive electrostatic forces, while at pH 1.6, it shrinks within a few minutes due to protonation of the carboxylate anions (23). This sharp swelling-deswelling behavior of the hydrogels makes them as suitable candidate for controlled drug delivery systems. Such on-off switching behavior as reversible swelling and deswelling has been reported for other ionic hydrogels(17,18).
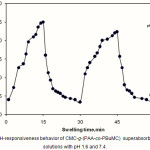 |
Figure 5: The pH-responsiveness behavior of CMC-g-(PAA-co-PBuMC) superabsorbing hydrogel in solutions with pH 1.6 and 7.4. Click here to View figure |
Thermo-sensitivity behavior of CMC-g-poly(AA-co-BuMC) Hydrogel
The effect of the temperature of the surrounding media on the water absorbency of the superabsorbents was investigated by varying the temperatures 20oC-40oC. As shown in Fig. 6, with increasing the solution temperature, the swelling capacity and rate increases. The maximum absorbency (129g/g) is obtained at 40 oC. The increase in rate and extent of swelling can be attributed to three factors that increase with temperature:
i) Flexibility of polymer chains ii) Diffusion of water molecules into superabsorbent backbones and iii) pH of surrounding media (Gibbs-Helmholtz equation),
higher pH enhances the hydrophilicity of the hydrogel, which inturn results in a stronger absorption of water [18].
Also,results indicates that the swelling and deswelling of the hydrogels were reversible. The response to temperature change is very quick. An abrupt decrease of swelling ratio is observed from 20oC to 40oC.
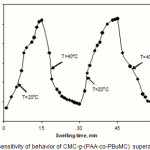 |
Figure 6: Thermo-sensitivity of behavior of CMC-g-(PAA-co-PBuMC) superabsorbing hydrogel. |
Standard calibration curve
The calibration curve of the absorbance as a function of the metronidazole concentration at 278 nm, shown in Figure 7, has a linear relationship with a correlation coefficient (r) of 0.979 and 0.995 at pHs 1.6 and 7.4, respectively.
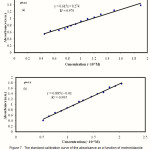 |
Figure 7: The standard calibration curve of the absorbance as a function of metronidazole concentration at 278 nm on the UV spectrophotometer at pH 1.6 (a) and pH 7.4 (b). |
Amount of drug encapsulated
The amount of metronidazole encapsulated in the polymeric hydrogels increased with increasing drug concentration . The amounts of the loaded drug in superabsorbent hydrogels was also significantly affected by the impregnation times (Figure 8). It is obvious that with increasing the loading time, the amount of drug loaded is initially increased and then begins to level off( in both pH=1.6 and 7.4). The initial increment in the amounts of the loaded drug with increasing the loading time can be ascribed to the increased drug diffusion into the swollen matrix(16). The most efficient time of loading efficiency in pH=7.4 was 65 h, where a major amount of drug (99%) was encapsulated.
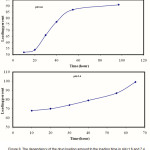 |
Figure 8: The dependency of the drug loading amount to the loading time in pH=1.6 and 7.4. |
In vitro release behavior of hydrogels
In order to simulate the possible effect of pH on drug release rate, a swelling study was conducted in simulated gastric fluid (pH 1.6) and simulated intestinal fluid (pH 7.4) at physiological temperature of 37 °C (Fig. 9). At pH 7.4, the hydrogel swells due to anion-anion repulsive electrostatic forces, while at pH 1.6, it shrinks within a few minutes due to protonation of the carboxylate anions. This swelling behavior of the hydrogels makes them as suitable candidate for designing drug delivery systems(27-29).
The most challenging task in the development of drug pharmaceuticals is to deal with instabilities of drugs in the harsh environment of the stomach. Drug encapsulation processes that require the use of organic solvents or heating might potentially physically modify or denature the therapeutic proteins. Encapsulation processes that require chemical bond formation among the encapsulation reagents might unintentionally chemically modify the therapeutic proteins. However, our drug loading process was desirable as the encapsulation of ephedrine was performed avoiding any organic solvent, high temperature, unfavorable pH and other harsh environmental conditions(30). The conditions were benign sufficiently as the resulting hydrogel physically entrapped the metronidazole drug. Fig. 9 shows the metronidazole release profile of the test hydrogels at pH 1.6 and subsequently at pH 7.4. The amount of metronidazole released at pH 1.6 was low; only about 18.7% metronidazole was released from the test hydrogel, whereas that released at pH 7.4 increased significantly (94%). The favorable metronidazole release performance could be attributed to the pH-sensitivity of the hydrogel. Swelling of such hydrogel in the stomach was minimal and thus the drug release was also minimal. Due to increase in pH, the extent of swelling increased as the hydrogel passed down the intestinal tract, the hydrogel swelled and the controlled release of metronidazole was affected. Fig. 9 shows the schematic of actuation at a distance and resultant squeezing effect for the pH-responsive CMC-based system. Because of the high matrix porosity of the hydrogel (Fig. 3), the capillary forces could reinforce the diffusion of solvent into the hydrogel; thereby the metronidazole release from the hydrogel matrix occurred mainly due to the diffusion of the drug though the pores of the swelled matrix in the intestinal pH.
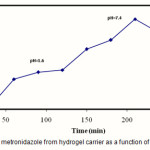 |
Figure 9: Release of metronidazole from hydrogel carrier as a function of time and pH at 37oC. |
Drug release mechanism
A simple semi-empirical equation has been introduced to express general drug release behavior depending on the geometry of a system (24):
![]()
where and Mt and M∞ are the absolute cumulative amounts of drug released at time t and after the finish of release respectively, k is a diffusional kinetic constant for the characteristics of a polymer network system, and n is a diffusional exponent representing the release mechanism.
When log Mt/M∞ is plotted against log t the value of n is obtained. The case of n=0.5 is for purely diffusion-controlled drug release (Fickian release) and the case of n=1 is for a drug release rate independent of time, corresponding to zero-order release kinetics (Case II transport). Other values for n are for anomalous transport kinetics and combined mechanisms of pure diffusion and Case II transport. In our experiments the diffusion coefficients were calculated based on fitting 25% and 70% of drug release, respectively. When 25% of drug had been released n was 9.83, indicating Case II transport close to zero order release. In the 70% case n was 0.69, related to non-Fickian or anomalous transport.
Conclusion
In the present study, CMC-g-(PNaAA-co-PBuMC) superabsorbent hydrogel was synthesized in an aqueous solution using a ceric ammonium nitrate initiator and a hydrophilic crosslinker. The swelling of hydrogel exhibited high sensitivity to pH-,-salinity and temperature. swelling-loss in salt solutions, in comparison with distilled water, can be attributed to charge screening effect and ionic crosslinking for mono- and multi-valent cations, respectively. The swelling capacity in CaCl2 is much lower than that in NaCl solution and distillated water.
Study of effect of H+/OH– concentration also carried out at various pHs shows that the swelling of hydrogel causes several large volume changes. So, we investigated the pH-sensitivity of the hydrogel. Ionic repulsion between charged groups incorporated in the gel matrix by an external pH modulation could be assumed as the main driving force responsible for such abrupt swelling changes.because this superabsorbent network intelligently responding to pH can be considered as an excellent candidate to design novel drug delivery systems, Therefore, we investigated the release behavior of metronidazole from this kind of responsive hydrogel. metronidazole was encapsulated as a model drug and in vitro release studies were carried out in SGF and SIF. The release value of drug from hydrogels at pH 7.4 was higher than that at pH 1.6 due to the electrostatic repulsion between carboxylate groups and the higher swelling capacity of the hydrogel.
References
- Buchholz, F. L.; Graham, A. T. Modern Superabsorbent Polymer Technology; Elsevier: Amsterdam, (1997).
- Peppas, L. B.; Harland, R. S. In Absorbent Polymer Technology; Elsevier: Amsterdam,( 1990).
- Po, R. J Macromol Sci Rev Macromol Chem Phys, C34, 607, (1994).
- Hoffman, A. S. In Polymeric Materials Encyclopedia; Salamone, J. C., Ed.; CRC Press: Boca Raton, FL,; Vol. 5, p 3282 (1996).
- Yazdani-Pedram, M.; Retuert, J.; Quijada, R. Macromol Chem Phys, 201, 923(2000).
- Sugahara, Y.; Takahisa, O. J Appl Polym Sci, 82, 1437(2001).
- Patel, G. M.; Trivedi, H. C. Eur Polym J, 35, 201 (1999).
- Silong, S.; Rahman, L. J Appl Polym Sci, 76, 516(2000).
- Kost, J. In Encyclopedia of Controlled Drug Delivery; Mathiowitz, E., Ed.; Wiley: New York, Vol. 1, p 445(1999).
- Peppas, N. A.; Mikes, A. G. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, Vol. 1, (1986)
- Fanta, G. F.; Burr, R. C.; Doane, M. W. ACS Symp Ser, 187, 195(1982).
- Yamaguchi, M.; Watamoto, H.; Sakamoto, M. Carbohydr Polym, 9, 15(1988).
- Rodehed, C.; Ranby, B. J Appl Polym Sci, 32, 3323(1986).
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Sung JJ, Chung SC, Ling TK. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori . N. Engl. J. Med. 332: 139–142(1995).
- Dollery CT. Testing and control of therapeutic materials and drugs. Acta Odontol. Venez.11: 28–38(1973).
- Silverstein, R. M.; Webster, F. X. Spectrometric Identification of Organic Compounds, 6th ed.; Wiley: New York, (1998).
- Castel, D.; Ricard, A.; Audebert, R. J Appl Polym Sci, 39, 11(1990).
- W. Xue, S. Champ, M. B. Huglin: New superabsorbent thermo reversible hydrogels. Polymer 2001, 42, 2247–2250.
- Lim, D. W.; Whang, H. S.; Yoon, K. J. J Appl Polym Sci, 79, 1423(2001).
- Lee, W. F.; Yuan, W. Y. J Appl Polym Sci, 77, 1760(2000).
- Park, S. E.; Nho, Y. C.; Lim, Y. M.; Kim, H. J Appl Polym Sci, 91, 636(2004).
- Burugapalli, K.; Bhatia, D.; Koul, V.; Choudhary,V. J Appl Polym Sci, 82, 217(2001).
- Siepmann, J, Peppas, N.A.Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 48: 139–57(2001).
- Lu, S.; Duan, M.; Lin, S. J Appl Polym Sci, 79, 1665(2001).
- Pourjavadi, A.; Sadeghi, M.; Hosseinzadeh, H. Polym Adv Technol, 15, 645(2004).
- Richter, A.; Bund, A.; Keller, M.; Arndt, K. Sens Actuators B, 99, 579(2004).
- Kiatkamjorwong, S.; Phunchareon P. J Appl Polym Sci, 72, 1349(1999).
- Wen-Fu, L.; You-Min, T. J Appl Polym Sci, 72, 1221(1999).
- Omidian, H.; Hashemi, S. A.; Sammes, P. G.; Meldrum, I. Polymer, 40, 1753(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









