Oxidative Cyclisation of 2'-Hydroxychalcones using Sodium Tellurite: Synthesis of Flavones
Surender Kumar and Dinesh Sharma
1Department of Chemistry, Technological Institute of Textile and Sciences, Bhiwani - 127 021, India.
2Department of Chemistry, BRCM College of Engineering and Technology, Bahal - 127 028, India.
A simple and very efficient method for the oxidative cyclisation of 2'-hydroxychalcones with sodium tellurite in dimethylsulphoxide has been developed which could provide a simple route for the synthesis of flavones and also the required compound obtained as the sole product in a very high yield.
KEYWORDS:Flavones; 2'-hydroxychalcones; sodium tellurite; dimethylsulphoxide; oxidative cyclisation
Download this article as:| Copy the following to cite this article: Kumar S, Sharma D. Oxidative Cyclisation of 2'-Hydroxychalcones using Sodium Tellurite: Synthesis of Flavones. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Kumar S, Sharma D. Oxidative Cyclisation of 2'-Hydroxychalcones using Sodium Tellurite: Synthesis of Flavones. Available from: http://www.orientjchem.org/?p=24694 |
Introduction
Flavones constitute a large sub group of naturally occurring flavonids and are widely distributed plant pigments1. Flavones occur in nature in Free State with varieties of substitution pattern. In recent years, much attention has been paid to the synthesis of flavones because of their various physiological and pharmacological properties .Flavones are known to be coronary dilator2 ,antiphogistic3 ,chloenic and histamine activity4 ,heart stimultant5,contol of cytotoxicity towards human nasopharynx carcinomacell 6,cancer preventive agents4 and regulate plant growth by inhabitation of exocytosis of the auxin indolyl acetic acid3.
Oxidative cyclisation of 2′-hydroxy chalcones constitutes an important route for the synthesis of flavones and number of oxidizing agents such as SeO27,8,DDQ9,10, Oxalic acid11,I2-DMSO12,Sodium periodate13 ,FeCl314etc.have been reported in literature for this conversion but these often require longer reaction time and formation of mixture of product contaning flavones, flavanones and aurones have been reported in some cases.
Material and Methods
All the chemicals were purchased from Aldrich and Fluka. Melting points were determined in open capillary tubes. IR (KBr) spectra were recorded in a Perkin-Elmer spectrum BX series FT- IR spectrophotometer and 1H NMR on Bruker Avance II 400 MHz instrument using tetramethyl-silane as an internal standard.
Experimental Procedure (General)
A solution of 2′-hydroxychalcone in dimethylsulphoxide and sodium tellurite were heated in round bottam flask with air condenser and calcium chloride guard tube in an oil bath at 130-400C for one hour .The completion of the reaction was checked on TLC. The reaction mixture was poured over crushed ice, stirred and extracted with ether and solvent removed by distillation. The residue was crystallized from aqueous methanol to give flavone.
Results and Discussion
Herein, we wish to report the oxidative cyclisation of 2′-hydroxychalcones with sodium tellurite in DMSO, which could provide an efficient and simple route for the synthesis of flavones and also the required compound obtained as a sole product in a very high yield.
2′-hydroxychalcone was heated with Sodium Tellurite in dimethyl sulphoxide at 130-40 0C under anhydrous conditions and starting compound was found to have to reacted completely after one hour when the reaction was checked on TLC (Scheme 1). On working up the reaction mixture, a colorless compound (m.pt. 96-970C) was obtained in 80% yield, which showed a singlet at δ 6.65 for one proton (H-3) and multiplet at δ 7.20-7.85 for eight protons along with a doublet at δ 8.10 for one proton (H-5) in its 1H-NMR.Based upon the above data the compound was identified as flavone. Using above procedure various substituted flavones were synthesized.
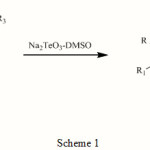 |
Scheme 1 |
Table 1: Synthesis of flavones
|
Entry |
R |
R1 |
R2 |
R3 |
m.p.( 0C) |
Lit m.p. (0C) |
% yield |
IR νC=O cm-1 |
1H-NMR (CDCl3) δ, ppm |
|
IIa |
H |
H |
H |
H |
95-96 |
96-9715 |
80 |
1620
|
δ6.65(s,1H,H-3),7.20-7.85 (bm,8H,C6H5,H- 6,H-7&H-8), and 8.10(d J=9.0Hz,1H,H-5) |
|
IIb |
H |
H |
H |
OCH3 |
156-57 |
157-5816 |
75 |
1625 |
δ3.90 (s,3H,OCH3),6.70 (s,1H,H-3 ), 7.0- (d,J=9.0Hz,2H,H-3’&H-5′),7.25-7.55(bm,3H,H-6,H-7&H-8),7.80 (d,J=9.0Hz 2H,H-2’& H-6′) and 8.20 (d J=9.0 Hz,1H,H-5) |
|
IIc |
OCH3 |
H |
H |
H |
108-10 |
11017 |
80 |
1630 |
δ3.95 (s, 3H, OCH3),6.70 (s,1H,H-3),6.90-8.0 (bm,7H, C6H5,H-6& H-8) and 8.10(d J=9.0Hz,1H,H-5) |
|
IId |
OCH3 |
H |
H |
OCH3 |
142-43 |
14518 |
70 |
1638 |
δ3.90(s,6H,2 x OCH3), 6.65 (s,1H, H-3),6.85-7.40 (m,4H,H-6,H-8, H-3’& H-5′),7.85 (d,J=9.0 Hz,2H, H-2’& H-6′) and 8.10(d,J=9.0 Hz,1H,H-5) |
|
IIe |
H |
CH3 |
H |
H |
120-21 |
12215 |
65 |
1625 |
δ2.45(s,3H, CH3),6.90 (s,1H,H-3),7.30 -7.90(bm,6H C6H5&H-8)and 8.0 (m 2H,H-5 & H-7 ) |
|
IIf |
H |
CH3 |
H |
OCH3 |
169-70 |
17018 |
70 |
1635 |
δ2.30 (s,3H, CH3), 3.75 (s,3H, OCH3),6.60 (s,1H, H-3),6.70(d J=9.0Hz,2H, H-3’& H-5′) ,7.30 (bs,2H,H-7&H-8),7.75(d =9.0Hz,2H,H-2′ & H-6′ )7.90.21(s,1H,H-5) |
|
IIg |
H |
H |
OCH3 |
OCH3 |
154-55 |
15619 |
70 |
1625 |
δ3.96&3.97(each s of 3H,2 x OCH3), 6.80 (s,1H, H-3),6.87-7.80 (m,6H,H-6,H-7, H-8,H-2′ H-5’& H-6′) and 8.10(d,J=8.0 Hz,1H,H-5) |
References
- Harborne J.B., Mabry J.J. and Mabry H., The Flavonoids, Chapman and Hall Ltd., London (1975).
- Brit D.F., Hendrich S. and Warg W., Pharnacology and Therapeutics, 90, 157, (2001).
- Havsteen B.H., The biochemistry and medical significance of the flavonoids, Pharmacology and Therapeutics, 96, 67, (2002).
- Geismann T.A. The Chemistry of Flavonoids Compounds, Pergemon Press, London (1962).
- Keflord and Chandr B.V., The Chemical constituents to Citrus Fruits, Academic Press, New York (1970).
- Benasson V., Jossany A., Tif A., Boda B. and Land J.,Radical Biology and Medicine, 27 (1), 95, (1999).
- Mahal H.S., and Venketaraman K., J.Chem. Soc., 569, (1936).
- Price W.A., Silva A.M.S. and Cavaleiro J.A.S., Heterocycles, 36, 2601, (1993).
- Mostahar S., Katun P. and Islam A., J. Biol. Sci., 7(3), 514, (2007)
- Imafuku K., Honda M. and Mcomie J.F.W., Synthesis, 2, 199, (1987).
- Zambare A.S., Sangshetti J.N., Kokare N.D. and Shinde D.B., Chinese Chem. Lett., 20, 171, (2009).
- Doshi A.K., Soni P.A. and Ghiya B.J., Indian .J.Chem. 25B, 759, (1986).
- Hans N. and Grover S.K., Synth.Commun., 23,1021, (1993),.
- Kumar V., M.Phil Thesis, Heterocyclic Compounds, M.D.University, Rohtak. (1992).
- Dann O.and Mylius G., Ann.Chem., 587, 1, (1954).
- Swapnil, S.R.; Pathan, M.Y.; Paike, V.V.; Pachmase, P.R.; Jadhav, W.N.; Pawar, R.P. , ARKIVOC, 2006, 2006, 43.
- Lee J.I., Son H.S., and Jung M.G., Bull. Korean Chem. Soc., 26(9), 1461, (2005).
- Fitzgorald D.M., Sullian J.F.O. Philbin E. M. and Wheeler T.S., J.Chem.Soc., 860, (1955).
- Hottori S., Bull.Chem.Soc.Japan, 2,171, (1927).

This work is licensed under a Creative Commons Attribution 4.0 International License.









