Influence of Myo-inositol on Thermodynamics of Clouding Behavior of Non-ionic Surfactant Tween-40
R.C. Chautmal1 and T.J. Patil2
1Department of Chemistry, GET’s Arts, Commerce and Science College, Nagaon, India. 2Department of Chemistry, Z.B. Patil College, Dhule, India.
Clouding behavior of non-ionic surfactant have been worked out in presence of Myo-ionositol in order to study the molecular interactions of additive on non-ionic surfactant.Tween-40 have been selected as surfactant while Myoi-inositol as an additive. Clouding of Tween-40 has been studied as a pure and in presence of additive at various concentrations. It has been found that below 0.1 Wt % of myo-inositol did not show marked effect on CP of surfactant.Considering CP as threshold temperature of solubility, the thermodynamic parameter of solubilization of pure surfactant and surfactant additive system has been evaluated. From this study, the process of clouding is guided by both enthalpy and entropy and overall process is exothermic.
KEYWORDS:Tween-40 (TW-40); Myo-inositol; Cloud point; Phase Separation Model.
Download this article as:| Copy the following to cite this article: Chautmal R. C, Patil T. J. Influence of Myo-inositol on Thermodynamics of Clouding Behavior of Non-ionic Surfactant Tween-40. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Chautmal R. C, Patil T. J. Influence of Myo-inositol on Thermodynamics of Clouding Behavior of Non-ionic Surfactant Tween-40. Available from: http://www.orientjchem.org/?p=24962 |
Introduction
The physico-chemical studies of additive surfactant solution have been created much interest regarding their pharmaceutical and industrial importance1-2. Non-ionic surfactant belonging to polyethylene oxide family, typically abbreviated as CiEj is widely used as detergents, solubilizer, emulsifier and pharmaceutical preparations; their practical importance has triggered a significant effort to gain the fundamental understanding of their micellization characteristics as well as their phase behavior in both aqueous and non-aqueous media3. The cloud point is important phenomenon of non-ionic surfactant, below CP a single phase of molecular solution exist, above CP water solubility of water surfactant is reduced and it results in to cloudy dispersion4-6, by formation of giant molecular aggregates in the state of separate phase7-8. The unique structures of surfactant offer a convenient way to study influence of additive like myo-inositol on micellization behavior through the clouding phenomenon supported by thermodynamic characterization using phase separation model.
Inositol is well studied organic compound with specific stereochemistry, its high reactivity control many cellular processes in living organism. Inositol is water soluble cyclic hexahydric alcohols. It has nine isomeric forms out of which myo-inositol is the only isomer which shows biological activity. Myo-inositol is crystalline compound with sweet test. It plays important role in animal and human metabolism. Myo-inositol is widely used for analytical as well as in pharmaceuticals, plant growing, food industry and variety of biotechnological processes. Myo-ino-sitol is key function in maintaining normal brain function.
In this paper the results of our study on clouding phenomenon of pure TW-40 in presence of myo-inositol have been reported. Considering cloud point as threshold temperature of solubility in aqueous medium, the thermodynamic parameters of clouding process ∆G0cl ,∆H0cl , ∆S0cl have been evaluated using phase separation model.
Materials and Methods
The nonionic surfactant Tween-40 (M.W. 1283.65) is product of SRL chemicals, India and myo-inositol (M.W. 180.16) is the product of Merck and used as received. Doubly distilled water with specific conductance 2-4 µs cm-1at 303.15 K was used in the preparation of all solutions of different concentrations.
The cloud point (CP) of surfactant solution was determined visually by noting the temperature at which turbidity was observed. The sample was then allowed to cool slowly under stirring conditions; the temperature of disappearance of turbidity was also noted. The average of the two was taken as cloud point of the system. The heating and cooling were regulated by less than 10C/min. around the cloud point. The reproducibility of the measurements is found to be within ±0.20C.
Molecular structure of clouding species and additive:
 |
Scheme 1 Click here to View scheme |
Results and Discussion
The cloud point of pure Tween-40 at various concentrations is given in Table-1. It was observed that cloud point increases with increases surfactant concentration, since at higher concentration well structured water-surfactant system is present. Rakshit et al9 pointed out that higher temperature is to be required to break the water-surfactant self assembly. It was found that below 1 Wt % there is mild variation in CP of pure surfactant, this might be due to the fact that to form cluster agglomerate of surfactant moiety are not sufficient at lower concentration. Table 1 shows the increase in CP values with increased surfactant concentrations. Higher temperature is required to remove the water molecules which are barriers for the micellar interaction. Once they move out at higher temperature the micelle-micelle interaction becomes easier. That is why the cloud point is seen at higher temperature. The variations of cloud points as a function of surfactant concentrations are shown in Fig.1.
Table 1: Cloud point of pure surfactant at different concentration.
| TW-40Wt % | Molarityx10-2 | Mole fractionsx10-4 | Cloud point0C |
| 1 | 0.7790 | 1.402 | 93.8 |
| 2 | 1.5581 | 2.804 | 94.3 |
| 3 | 2.3371 | 4.205 | 95.0 |
| 4 | 3.1161 | 5.606 | 95.3 |
| 5 | 3.8951 | 7.006 | 95.6 |
TW-40 / Myo-inositol system:
The influence of additive on CP of Tween-40 at varied concentration has been studied. The results of mixed system are presented in Fig.-2. It was found that below 0.1 Wt % of myo-inositol did not show marked effect on CP of surfactant, since at lower concentration surfactant moiety do not agglomerate into visible micelle. The CP values declined with increased additive concentration effectively. This is mainly due to removal of water by the additive which helps the surfactant moiety to come closer to each other resulting in to phase separation by cloudy dispersion. Here additive compete for the water molecule with the micelles and the surfactant becomes less hydrated and resulting into lowering of cloud point.
![Figure 1: Variation of CP as a function of [TW-40]](http://www.orientjchem.org/wp-content/uploads/2011/06/Vol27_Iss2_Cha_Inf_Fig1-150x150.jpg) |
Figure 1: Variation of CP as a function of [TW-40] Click here to View Figure |
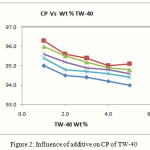 |
Figure 2: Influence of additive on CP of TW-40
|
Thermodynamics of clouding phenomenon:
Cloud point is characteristics of non-ionic surfactants. The desolvation of hydrophilic group of surfactant leads to phase separation and visibility observed as cloudy dispersion. Kjellander et al10 reported that phenomenon of clouding is entropy dominated. At the CP, the water molecule gets totally detached from micelles. Considering the cloud point as a separation point, the thermodynamic parameter such as standard free energy (∆G0cl), enthalpy (∆H0cl ), and entropy (∆S0cl ) for clouding ›process have been evaluated using phase separation model11. Standard free energy (∆G0cl) evaluated using relation-
ΔGocl = -RT ln Xs (1)
Where Xs is the mole fractional solubility of the solute.
Standard entropy (ΔSocl) for the clouding process have been calculated using following relationship-
ΔSocl = (ΔHocl – ΔGo cl ) / T (2)
The standard enthalpy (ΔHocl) for clouding process can be calculated from the solublization curve is given by
– ΔHocl = RT2 (d ln Xs/ dT)
Ln Xs = (ΔHocl/T)(1/T) + C (3)
The negative value of ΔHocl indicate that process of clouding is exothermic in nature. The thermodynamic parameters of clouding for pure TW-40 are given in Table-2
Table 2: Thermodynamic parameters of solublization of TW-40
| TW-40Wt % | ∆G0clkJmol-1 | -∆H0clkJmol-1 | -∆S0clJ mol-1K-1 |
| 1 | 27.06 | 186.9 | |
| 2 | 24.98 | 181.0 | |
| 3 | 23.79 | 41.5 | 177.4 |
| 4 | 22.92 | 174.9 | |
| 5 | 22.26 | 173.0 |
Table 3: Thermodynamic parameters of TW-40 in presence of Myo-inositol
| Myo- inositolWt % | ∆G0clkJmol-1 | -∆H0clkJmol-1 | -∆S0clJ mol -1K-1 |
| 0.1 | 24.25 | 43.2 | 183.12 |
| 0.2 | 24.24 | 63.8 | 239.0 |
| 0.3 | 24.22 | 79.5 | 281.7 |
| 0.4 | 24.20 | 80.4 | 284.5 |
| 0.5 | 24.18 | 81.3 | 287.1 |
From these values it is indicated that overall entropy is very high and hence the free energy change is more negative and appearance of cloud point is facile. The thermodynamic parameters of mixed system are given in Table-3. ∆H0cl < ΔGocl indicating the process of clouding is exothermic and also ∆H0cl > T∆S0cl indicating that the process of clouding is guided by both enthalpy and entropy.
The present work would be supportive evidence for the probable interaction between nonionic surfactant and biomolecule leading to phase separation at cloud point.
Acknowledgement
The author (Chautmal R.C.) thankful to University Grant Commission, WRO, Pune for financial assistance under Minor research project F. No. 47-905/09 (WRO) dated. The author is also thankful to, Hon’ble Principal Dr. P.N.Patil, GET’s Arts, Comm. and Science College, Nagaon, Dist., Dhule. Hon’ble Principal, Z.B. Patil College, Dhule. Head, Department of Chemistry, Z.B.Patil College, Dhule and Head, Department of Chemistry, GET’s Arts, Comm. and Science college, Nagaon, Dist., Dhule for providing laboratory facilities.
References
- Jones M.N., J.colloid Interface Sci. 23, 36 (1967)
- Goddard E.D., Colloids Surf. 19, 255 (1986)
- Goddard E.D. and Anantpadmanaphan K.P., CRC press Boca Rotan PL (1993) p 123
- Myers D., Surfactant science and Technology 2nd Edition VHC, New York, (1963)
- Patil T.J., Patil H.A., Int.J. of Chem. Sci. 3(4), 751-755(2005)
- Patil T.J., Patil H.A., Int.J. of Chem. Sci. 3(3), 507-512(2005)
- Shinoda K.,Nakagawa T.,Tamamushi B. and Semura T.I., Colloidal Surfactant, some Physicochemical properties, Academic press, New York (1963)
- Blankchtein D., Thurston and Benedeck G.B., J. Chem. Phy. 85, 7268 (1986)
- Koshi L., Saiyyad A.H. and A.K. Rakshit J. of Colloid Polym. Sci 274, 582 (1996)
- Kjellander R. and Florin E., J. Chem. Soc. Faraday Trans I, 17, 2053 (1981).
- Attwood D. Florence A.T., Surfactant system Champman and Hall, London (1933) P 99

This work is licensed under a Creative Commons Attribution 4.0 International License.









