Column Method: an Analytical Approach for the Removal of Chromium by Using iron Oxide Nanoparticles
Yogendra Singh1, Inderjeet Singh2 and Niranjan Lal1
1Department of Chemistry Vidya College of Engineering, Baghpat Road, Meerut - 250 005 India. 2Department of Chemistry CCRD,College Muzaffarnagar India.
Nanoparticles are being intensively investigated because of their unique optical, electrical and catalytic properties which make them a potential material for utilization in the field of medicine electronic and environmental issues. This paper is concerned with the removing a heavy metal, Chromium, from its aqueous solution by Iron oxide nanoparticle filtration. Solutions of varying chromium concentrations (50-250ppm) were prepared and passed through a column of Iron oxide nanoparticle. Effluent samples collected at different column depths were analyzed for the concentration of Chromium ions using an Atomic Absorption Spectrometer. The injection rate and pH of the influent solution were also varied to study their effects on the Chromium removal efficiency by Iron oxide nanoparticle. A series of curves were constructed to determine the capacity of Iron oxide nanoparticle column at various depths. The removal efficiency was found to be in the range 99-100%. These high removal efficiencies were likely attributed to the strong affinity of chromium ions to the surface of Iron oxide nanoparticle particles. It can be concluded that due to their relatively high adsorption capability, Iron oxide nanoparticle has the potential to be utilized for the removal of heavy metals from water and waste water.
KEYWORDS:Nanoparticles chromium; Toxic metals; wastewater; adsorption
Download this article as:| Copy the following to cite this article: Singh Y, Singh I, Lal N. Column Method: an Analytical Approach for the Removal of Chromium by Using iron Oxide Nanoparticles. Orient J Chem 2011;27(2). |
| Copy the following to cite this URL: Singh Y, Singh I, Lal N. Column Method: an Analytical Approach for the Removal of Chromium by Using iron Oxide Nanoparticles. Orient J Chem 2011;27(2). Available from: http://www.orientjchem.org/?p=24936 |
Introduction
Chromium (Cr) is a transition element located in the group VI-B of the periodic table with a ground-state electronic configuration of Ar 3d54s1. The stable forms of Cr are the trivalent Cr (III) and the hexavalent Cr (VI) species (Adriano DC, et al). Chromium (III) is an essential nutrient that helps the body use sugar, protein, and fat. (Mertz et al, Walter et al) And it is a very stable oxidation state for chromium. In this state, the chrome is labile and kinetically very slow to react or form complexes. It is not a strong oxidizer and the human’s natural body acidity is enough for the chrome to keep to this Cr (III) state and the hexavalent Cr (VI) species, cause breathing high levels of chromium (VI) can cause irritation to the nose, such as runny nose, nosebleeds, and ulcers and holes in the nasal septum (Cronin et al). the large amount of Chromium is very harmful and it is responsible for many allergic and many other harmful disease (ATDSR report). The harmfulness and hazardous effect is also depend upon the oxidation state of chromium and it has been find that Cr (VI) is more harmful than Cr(III) (Becquer et al., 2003).Nanotechnology, which is sometimes shortened to “Nanotech”, refers to a field with structures of the size 100 nanometers or smaller, and involves developing materials or devices within that size. Nanotechnology is an important technology which extension is very large in every field due to the large surface to volume ratio nanoparticles becomes vital and many properties become specific. (Lehn M. et al, 1998,2000), to developing new materials with dimensions on the nanoscale, even to speculation on whether we can directly control matter on the atomic scale(BK Pong et al, Vikas Berry et al ) There has been much debate on the future of implications of nanotechnology. Nanotechnology has the potential to create many new materials and devices with wide-ranging applications, such as in medicine, electronics, and energy production (Spatz JP,et al ,Trindade T et al,,Zheng et al,). These concerns have led to a debate among advocacy groups and governments on whether special regulation of nanotechnology is warranted. Therefore here we are using the removal of toxic and heavy metal ions by applying Iron oxide nanoparticles. Many research has been shown that if any aqueous solution has the concentration of metal is beyond certain limits then the metal become toxic and solution become toxic but it has recommended by the WHO and other pollution control authority the industries has to treat the water prior to discharge other due to leaching tendency of soil it will pollute to the ground water pollution.
cementation, (M. H. El-Awady et al, Vallejo B et al ) reverse osmosis,( A Ameri1 et al) membrane filtration (R. Sondhi, et al)ion exchange, adsorption (P. Rana et al ,Srivastava HPC et al) and co-precipitation (ELLIS at al) But these methods were not very effective for a long time .Nanoparticles owe their characteristics in the removal of toxic metal very effectively due to large surface to volume ratio and the span life of these nanoparticles is very high (Singh Yogendra et al). Iron oxide nanoparticles has a vital role in the medical areas as well as another field also (Laurent, Sophie et al)
Materials and Methods
First phase of this study, the mean concentration of Cr was inspected in the effluents discharged from electroplating industries and it was found that this figure was about 200 mg/L, thus this concentration had been chosen as the initial amount for treatment in our Column system. For starting the work distilled water had been passed from the different height of Iron oxide nanoparticles column to determine the initial flux in various pressures of 100, 120, 150, 170 and 200 psi at an ambient temperature of 25 ºC. Then, the column was operated to determine the optimized applying pressure. At last, the optimum amounts of temperature, pH, concentration flow rate, and had been investigated at the optimum pressure. In the next phase of the study, the amount of flux which could pass from the Iron oxide Nanoparticles had been measured. The efficiency of treatment by Iron oxide nanoparticles (Cr removal efficiency) was then determined by the following equation.
Cr Removal Efficiency= C1-C2/C1X100
Where C1= initial Cr concentration
C2=, Cr concentration after treatment
Experimental procedure
Materials
All the materials were used without further purification. Iron chloride hexahydrate (FeCl3·6H2O, N99.0%), sodium oleate (N90%), and Isopropyl alcohol (95%) were from E Merck. (India), and n-hexane (N 99.5%) and 28 wt.% aqueous ammonia solution were obtained from Hi media India , Ltd. (, India).
Preparation of iron oleate/n-heptane mixture
First, a mixture of iron oleate and n-hexane was prepared in a as described as. For the synthesis of 2.8×10−1 mol/L iron oleate in n-hexane, 25 mmol of FeCl3·6H2O and 55 mmol of sodium oleate were dissolved in a redispersed in a solution of n-hepane and water. The content of α-Fe2O3 nanoparticles in hexane is 0.5 wt.%. mixture of 30 mL distilled water, 40 mL Isopropyl alcohol , and 70 mL n-hexane . For the synthesis of 2.8 mol/L iron oleate dissolved in n-hextane , 50 mmol of FeCl3·6H2O, 150 mmol of sodium oleate, 30 mL of distilled water, 40 mL of ethanol, and 20 mL of n-heptane were used. The solution was heated at 343 K for 4 h with mild mixing. After the mixing procedure, the dark upper organic layer was washed three times with 15 mL distilled water and was subsequently collected by using a separatory funnel.
Synthesis of α-Fe2O3 nanoparticles:
For the synthesis of α-Fe2O3 nanoparticles, a 28-wt. % aqueous ammonia solution was slowly titrated into the asprepared mixture of iron oleate and n-hexane at 303 K with stirring. The additive content of the aqueous ammonia solution was 3.64 g and 9.10 g for the 2.8×10−1 mol/L and 2.8 mol/L iron oleate solutions. After the titration, the synthesis solution was heated to 303 K, 343 K, or 373 K and maintained at that temperature for 24 h with stirring. The prepared nanoparticles were precipitated by adding an excess amount of Isopropyl alcohol and separated by centrifugation.
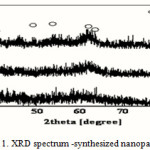 |
Figure 1: XRD spectrum -synthesized nanoparticles Click here to View figure |
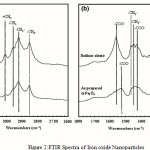 |
Figure 2:FTIR Spectra of Iron oxide Nanoparticles |
Preparation of column
To carry out the experimental investigation, a laboratory scale Iron oxide nanoparticles Filter was designed and fabricated. The filter had a circular cross-section with a diameter of 60 mm and a total height of 1.5m. It was filled with Iron oxide nanoparticles to a depth of 0.8m to 2.0m to leave some empty space for pouring the solution. Sampling taps were fitted at 0.4 and 0.8m depth to investigate the effects of Iron oxide nanoparticles depth. Four different influent injection rates of 10 to 60 ml/minute were considered to study the effects of injection rates on the Chromium removal efficiency, Prior to filling the column, the ordinary iron oxide nanoparticles was thoroughly washed with a substantial amount of water to remove clay particles. The standard was made by using Chromium(III) Chloride. First we prepared 1000 ppm solution and other low concentration solutions were obtained by further dilution .The filter column was completely filled with the iron oxide nanoparticles overhead tanks with firstly, synthetic solutions of different concentrations prepared in the laboratory and then with an actual industrial effluent. A Peristaltic Pump attached to the overhead tank controlled the injection rate. Small volumes of effluent were collected from all the sampling ports at regular time intervals. An Atomic Absorption Spectrometer (Thermo Electron corporation,UK Made) was utilized to determine the concentration of Chromium ions in the effluent samples.
Results and Discussions
The selection of the pH values was done on the basis of the appropriate reduction in concentration condition of industrial effluents. Solutions of all concentrations were prepared at two different pH values, 4 and 10 . For a particular injection rate, the effect of pH could then be compared for each and every concentration.
Table 1: Relative removal of concentration of metal (at pH =4), Column height 0.8m, Flow rate 60 ml/min
| Concentration of metal | % Removal of metal |
| 10 | 90.56 |
| 50 | 87.32 |
| 100 | 84.38 |
| 150 | 81.21 |
| 200 | 79.02 |
| 250 | 75.35 |
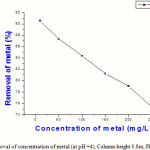 |
Figure 3: Relative removal of concentration of metal (at pH =4), Column height 0.8m, Flow rate 60 ml/min |
Effect of Influent Concentration on the Removal Efficiency at an injection rate of 10ml/minute to 60 ml/min and a pH In this study, it was found that the removal of Chromium is slightly greater at a pH of 10 (table 2,fig 2) as compared to a pH of 4. This difference increases with increasing concentration of chromium in the influent solution e.g. at a flow rate of 10ml/minute to 60 ml/minute and an initial concentration of 50ppm, the removal is 100% at both pH values; whereas at a concentration of 250ppm 90.56% of chromium is removed at a pH of 4 and 100% at a solution pH of 10.This precipitate, although not permanently adsorbed by the Iron oxide nanoparticles particles, is nevertheless retained/trapped in the Iron oxide nanoparticles column. However, washing of the Iron oxide nanoparticles will definitely remove the hydroxide and bring the chromium into direct contact with the external environment. The maximum removal of chromium was obtained at pH-10 therefore Aqueous become alkaline so
Prior to the discharge effluents its pH must be reduced to 7, otherwise the effect which may lead to toxicity may be observed.
Table 2:Relative removal of concentration of metal (at pH =10),Column height 0.8m,Flow rate 60 ml/min
| Concentration of metal(ppm) | % Removal of metal |
| 10 | 94.56 |
| 50 | 90.32 |
| 100 | 87.38 |
| 150 | 85.21 |
| 200 | 83.02 |
| 250 | 82.35 |
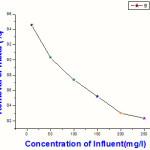 |
Figure 3a: Relative removal of concentration of metal (at pH =10), Column height 0.8m, Flow rate 60 ml/min |
Fig.1 shows the effect of concentration on removal efficiency and it has been observed that as concentration of metal is increasing then removal efficiency of nanoparticles is decreasing therefore nanoparticles has high efficiency at low concentration. The industrial sample analyzed in this study had a concentration of 230 ppm. Maximum removal was observed for an influent concentration of 10ppm at all injection rates. Even at a very high concentration of 250mg/L, the removal efficiency rarely falls below 80%.. For example, a concentration of 200ppm will have a higher surface loading as compared to a concentration of 100ppm. The number of ions coming into contact with the Iron oxide nanoparticles in the same amount of time increases. Since the number of available adsorption sites is constant for all concentrations, at higher influent concentrations, more ions go through without being adsorbed. Fig.3 demonstrates the effect of influent injection rates at various column depths. Generally, the removal efficiency of the Iron oxide nanoparticles column decreases with increasing injection rate of the influent solution. This effect is more pronounced for shallower columns and less for deeper ones. For example, when the column depth is only 0.8m, the removal efficiency decreases from 100% to 94.56 % (Table 3,figure 3) when the injection rate is increased from 10 to 60 ml/min.On the other hand, for a 1.2m deep column, the variation is a mere 2% for an identical range of injection rates. As expected, the effect of injection rate on the performance of the middle column (1.4 m depth) is somewhere in between. Fig.3 summarizes the variation of chromium removal efficiency with the depth of Iron oxide nanoparticles column at a specific injection rate. The results show that, deeper the column, greater the chromium removal capacity. Even after the passage of 100 liters of influent solution, the removal efficiency remains at 100% for a column 1.8m deep,pH=10,(Figure 4,Table 4). As height of column is increased then the removal of metal is decreasing at the height at 0.8 m the removal tendency was recorded 94.56. Similar results were obtained for an actual industrial effluent sample, which had a concentration of 270ppm and a pH of 10. Effect of Influent Injection Rate and Column Depth on Removal Efficiencies This behavior is attributed to the early exhaustion of the shallower column. Lesser amount of Iron oxide nanoparticles a means presence of large adsorption sites; therefore, the passage of even a small volume of solution results in those sites being taken up.
Table 3 : Removal of metal at pH=10 ,with altering height of column, Flow Rate 60 ml/min
| Height of column (in meter ) | % Removal of metal |
| 0.8 | 94.56 |
| 1 | 95.38 |
| 1.2 | 96.31 |
| 1.4 | 96.68 |
| 1.6 | 97.10 |
| 1.8 | 97.56 |
| 2 | 97.99 |
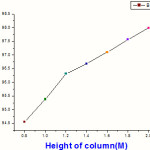 |
Figure 4:% Removal of metal at pH=10 ,with altering height of column, Flow Rate 60 ml/min |
For the removal of cromium the different heighten column were made which height were on vary from 0.8 m to 1.2 m..However, it’s quite significant on the shallowest column; the efficiency decreases by 26% when the rate is increased from 10 to 60 ml/min.To determine the capacity of Iron oxide nanoparticles column, the 250 ppm solution was passed through the column till the Chromium concentration in the effluent became equal to that of the influent. This is the stage when the Iron oxide nanoparticles surface is completely exhausted and no more adsorption sites are available. Fig. 3 reveals the exhaustion limit or the capacity for the three different depths.0 0.8m to 1.8 m depth.
Table 4: Removal of metal at pH 10, column height 1.8 cm with discernable flow rate
| Flow Rate(ml/min) | %Removal of metal |
| 10 | 100 |
| 15 | 100 |
| 20 | 100 |
| 25 | 94.5 |
| 30 | 92 |
| 35 | 90 |
| 40 | 88 |
| 60 | 74 |
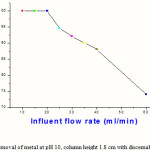 |
Figure 5:Removal of metal at pH 10, column height 1.8 cm with discernable flow rate |
Conclusions
Iron oxide nanoparticles acts a Filters are very effective in removing Chromium from wastewater even of very high concentration e.g. 250 mg/L The solution pH does not have a significant impact on the removal efficiency. Since at higher pH it has been recorded the precipitation occurs and these precipitate should break prior to discharge the solution this is only possible after to acidify the solutioion. The removal efficiency of Chromium decreases as the flow rate increases. Although Iron oxide nanoparticles is quite effective even at a rate of 20 ml/minute, with column height 1.8 m and pH=10 is strongly recommended The removal efficiency increases with the depth of the Iron oxide nanoparticles column. Although it’s quite high even at a depth of 1.8m , best results are obtained for a 1.8m deep column. Iron oxide nanoparticles Filters are very effective in removing heavy metals.
References
- .Adriano DC. Trace Elements in the Terrestrial Environment. New York7 Springer Verlag; 1986. p. 105– 23.
- Mertz, Walter “Chromium in Human Nutrition: A Review“. Journal of Nutrition 123 (4): 626–636.
- Cronin, Joseph R. (2004). “The Chromium Controversy”. Alternative and Complementary Therapies 10 (1): 39–42
- “Chromium”. Agency for Toxic Substances & Disease Registry, Centers for Disease Control and Prevention. February 2001. Retrieved on 2007-10-02.
- .Lehn, J.-M (1988). “Perspectives in Supramolecular Chemistry-From Molecular Recognition towards Molecular Information Processing and Self-Organization”. Angew. Chem. Int. Ed. Engl. 27 (11): 89–121.
- .Lehn, J.-M (1990). “Supramolecular Chemistry-Scope and Perspectives: Molecules, Supermolecules, and Molecular Devices (Nobel Lecture)”. Angew. Chem. Int. Ed. Engl. 29 (11): 1304–1319. doi:10.1002/anie.199013041
- .Lehn, J.-M.. Supramolecular Chemistry: Concepts and Perspectives. Wiley-VCH. ISBN 978-3-527-29311-7
- .BK Pong et al. New Insights on the Nanoparticle Growth Mechanism in the Citrate Reduction of Gold(III) Salt: Formation of the Au Nanowire Intermediate and Its Nonlinear Optical Properties J. Phys. Chem. C, 111 (17), 6281 -6287, 2007.
- Vikas Berry, Ravi F. Saraf “Self-Assembly of Nanoparticles on Live Bacterium: An Avenue to Fabricate Electronic Devices Angewandte Chemie International Edition Volume 44, Issue 41 , Pages 6668 – 6673 2005 .
- .Spatz JP,Mossmer S and Hartmann C 2000 lanmair 16,407.
- ..Trindade T,Obrein P and Oickett NL 2001
- Vallejo B., Munoz R., Izquierdo A., Luque de Castro M.D. Cement for stabilization of industrial residues containing heavy metals. Journal of Environmental Monitoring 1999, 1 (6), pp.563-568
- .Zeng L. Preliminary Study of Multiple Heavy Metal Removal Using Waste Iron Oxide Tailings.Proceedings of the Remediation Technologies Symposium, October 16-18 2002, Banff, Alberta
- Knocke W.R., Hemphill L.H. chromium (II) World Health Organization, Geneva, Guidelines for Drinking Water Quality, 1984 sorption by waste rubber.15. Marta erná “Use of solvent extraction for the removal of heavy metals from liquid wastes” Environmental Monitoring and Assessment Volume 34, Number 2 / January, 1995 pp151-162
- 16. Geckeler K.E., Volchek K., “Removal of Hazardous Substances from Water Using Ultrafiltration in Conjuction with Soluble Polymers”, Critical Review, Environmental Science and Technology, 30(3), 1996
- M. H. El-Awady,1 T. M. Sami2 Removal of Heavy Metals by Cement Kiln Dust Bull. Environ. Contam. Toxicol. (1997)V 59 : pp 603-610 .
- A Ameri1, *M Gholami 1, F Vaezi 2, M Rahimi1, M ahmodi1,BMoosavi1, “Application and Optimization in Chromium-Contaminated,Wastewater Treatment of the Reverse Osmosis Technology” Iranian J Publ Health, Vol. 37, No.3, 2008, pp.77-84
- R. Sondhi, Y. S. Lin, and F. Alvarez “Crossflow filtration of chromium hydroxide suspension by ceramic membranes: fouling and its minimization by backpulsing” Journal of Membrane Science,Volume 174, Issue 1, 20 July 2000, Pages 111-122
- .P. Rana, N. Mohan and C. Rajagopal, Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes, J .
- References and further reading may be available for this article. To view references and further reading you must purchase this article.
- Water Research,Volume 38, Issue 12, July 2004, Pages 2811-2820.
- .Srivastava HPC, Mathur RP, Mehrotra I (1986) Removal of chromium from industrial effluents by adsorption on sawdust. Environ Technol Lett 7:55–63
- ELLIS, K.V. Slow Gold nanoparticles Filtration. Critical Reviews in Environmental Control 1985, 15, pp.315-354.
- .Yogendra Singh ,Aditya Gautam,Vikesh Kumar “ Toxic cadmium is curse for living being :Develop the method to remedy cadmium toxicity problem from industrial effluents by using gold nanoparticle.Environment conservation journal 9(3),117-124,2008.(ISSN 0972-3099).
- Laurent, Sophie; Forge, Delphine; Port, Marc; Roch, Alain; Robic, Caroline; Vander Elst, Luce; Muller, Robert N. (2008). “Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications”. Chemical Reviews 108 (6): 2064.
- .Jianling Zhang, Jimin Du, Buxing Han, Zhimin Liu, Tao Jiang, Zhaofu Zhang Angewandte Sonochemical “Formation of Single-Crystalline Gold Nanobelts” Chemie International Edition Volume 45, Issue 7 , Pages 1116 – 1119 2006 .
- Yogendra Singh,inderjeet Singh,Pankaj Kumar, “Fabrication of Iron oxide nanoparticles :An overview”proceeding of seminar on “Nanoscience and Nanotechnology” 27-28 February 2009,MS college Saharanpur pp 88-91

This work is licensed under a Creative Commons Attribution 4.0 International License.









