Volatile Components and Antioxidant Effect of Essential Oil of Anthemis mauritiana Maire and Sennen Flowers
Ahmed Karim¹, Jean-Paul Wathelet², Hicham Harnafi³, Souliman Amrani³, Ahmed Melhaoui, Mostafa El-Achouri¹ And Mohammed Aziz¹
¹Laboratory of Physiology and Ethnopharmacology, Mohammed First University, Sciencies Faculty BP 717, 60000 Oujda, (Morocco). ²Laboratory of Biochemistry, Mohammed First University, Sciences Faculty, BP 717, 60000, Oujda, (Morocco). ³Laboratory of Organic and General Chemistry, Liege University, Gembloux Agro-Bio Tech, Passage des Déportes, 2, BP 5030, Gembloux, (Belgium).
The volatile components isolated from flowers of Anthemis mauritiana have been studied. The essential oil was obtained by hydrodistillation and by micro steam distillation and analysis were performed by GC/MS. The main constituents of the essential oil obtained by both methods were α-pinene, β-pinene, β-myrcene, γ-terpinene respectively by hydrodistillation and micro steam distillation. The essential oil obtained by hydrodistillation was also subjected to screening for its possible antioxidant activity by using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The best result was obtained at a dose of 100µg/ml with an inhibition of 37.80 ± 0.24 %, this activity was less effective than the synthetic BHT with 56.59 ± 0.43% at the same dose.
KEYWORDS:Anthemis mauritiana; Essential oil; Antioxidant activity; GC/MS
Download this article as:| Copy the following to cite this article: Karim A, Wathelet J. P, Harnafi H, Amrani S, Melhaoui A, El-Achouri M, Mohammed Aziz M. Volatile Components and Antioxidant Effect of Essential Oil of Anthemis mauritiana Maire & Sennen Flowers. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Karim A, Wathelet J. P, Harnafi H, Amrani S, Melhaoui A, El-Achouri M, Mohammed Aziz M. Volatile Components and Antioxidant Effect of Essential Oil of Anthemis mauritiana Maire & Sennen Flowers. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=11643 |
Introduction
The genus Anthemis (Asteraceae, syn. Compositae) is the second largest genus in this family, with more than 210 species that occur in the Mediterranean region, southwest Asia and eastern Africa (1). Anthemis mauritiana Maire & Sennen is an endemic specie distributed in Morocco and Algeria. The species of this genus are widely used in the pharmaceutic, cosmetic and food industries. The flowers of the genus have been well-documented. Their main components are natural flavonoids and essential oils (2, 3). In the Mediterranean region, tinctures, tisanes, and salves of this genus are widely used as anti-inflammatory, antioxidant, antibacterial, and antispasmodic agents (4-7).
In the present study, we were interested in evaluating the composition and the possible antioxidant activity of Anthemis mauritiana Maire & Sennen flowers (EOAM) essential oil. Indeed, many pathological conditions are associated with oxidative stresses which are responsible for the development of numerous diseases such as cancer and cardiovascular complications (8, 9, 10). Nowadays, there is considerable evidence that the Mediterranean diet, rich in fruits, vegetables and natural anti-oxidants is able to prevent the risk of oxidative stress related diseases (11).
Materials and methods
Chemicals
Butylated hydroxytoluene (BHT), 2,2- diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich , and homologous series of C7_C30 n-alkanes and various reference chemicals used for identification were obtained from Supelco Analytical (Bellefonte, USA). Diethyl ether and methanol used in this study were purchased from Merck (Darmstadt, Germany).
Plant material
The flowers of Anthemis mauritiana Maire & Sennen were collected locally in May 2009 from the North eastern area of Morocco; the botanical identification was done by Professor Benyounes Haloui at the department of Biology, Faculty of Science University Mohammed I Oujda, Morocco. A voucher specimen (N° 64666) was previously deposited at the Scientific Institute of Rabat.
Isolation of the essential oil
Hydrodistillation: The air-dried and ground flowers (100g) of Anthemis mauritiana were submitted for 3 h to water distillation using a Dean stark type apparatus (yield: 0.2%).
Micro-steam distillation-extraction method: 35 g of Anthemis mauritiana Maire & Sennen flowers were subjected to a simultaneous distillation-extraction method for 1 hour using a Likens-Nickerson apparatus with 1 ml of diethyl ether as solvent.
Gas Chromatography Mass Spectrometry (GC/MS) analysis
GC/MS analysis of the essential oil was performed using Agilent-Technologies 6890N Network gas chromatographic (GC) system (Agilent-Technologies, Little Falls, CA, USA) equipped with a flame ionization detector (FID), a Varian CP-99927 capillary column (30 m _ 0.25 mm, film thickness 0.25 µm) and an Agilent Technologies 5975 inert XL mass detector (electron impact mode). The injector and detector temperatures were set at 230 and 250 °C, respectively. Column temperature was programmed from 40°C to 260 °C at a rate of 10 °C/min. Helium was used as carrier gas at a flow rate of 1.2 ml/min. A sample of 1.0 µL of the essential oil diluted 2000 times with diethyl ether was injected using the splitless mode
Compounds identification.
The identification of the essential oil constituents was based on a comparison (i) of their retention factors to those of (C7_C30) n-alkanes and (ii) of their Kovats indices to published data and to those of reference compounds. The molecules were further identified and authenticated using their MS data and the Wiley 275.L mass spectral library.
DPPH scavenging activity
The scavenging activity of essential oil against DPPH (2,2-diphenyl-1-picrylhydrazyl) radical was measured according to the method of Blois (12). 4 ml of a 0.1 M DPPH methanolic solution was added to 1 ml of essential oil solubilized in methanol. A series of concentrations ranging from 10 to 100 mg/ml of essential oil extracted by hydrodistilation were tested. The mixtures were shaken vigorously and incubated in the dark for 30 min. The reduction of DPPH absorption was then measured at 517 nm. The scavenging activity of DPPH radical (%) was calculated following the equation:
100 x (A517blank – A517sample) / A517blank.
Where A517blank is the absorbance of the control reaction (a reaction with all the reagents except the essential oil tested) measured at 517 nm, and A517sample is the absorbance of the essential oil sample measured at 517 nm against the corresponding blank. Tests were carried out in triplicate.
Results
The essential oil samples obtained by both methods were compared. In the case of hydrodistillation, 45 compounds were identified representing, the 90.84% of the total oil, while in the micro-steam distillation method, 49 compounds were identified representing 77.56% of the oil. The results show that the percentage of each compound depend of the extraction method. Some molecules are more abundant in the essential oil extracted by hydrodistillation than in the essential oil extracted by micro-steam distillation with respectively 28.14 % and 15.36 % for α-Pinene, 15.57 % and 7.81% for β-Pinene, 4.75 and 0.34% for β-Myrcene, 4.28 and 0.78% for γ-Terpinene, 5.76 and 3.27 % for α-Curcumene, 3.64 and 0.72% for β-Caryophylene, and 2.84 and 0.54% for D-Germacrene. On the other hand, some compounds are found most abundantly in the oil extracted by micro-steam distillation than in the oil extracted by hydrodistillation. For example, this is the case of lavandulol with 2.39% and 0.19%, sabinol acetate (acetyl) 6.04% and 0.65%, heptacosane 2.06% and 0.3% respectively. Some molecules are only found in essential oils extracted by micro-steam distillation such as octanoic acid and linoleic acid, others such as camphene and limonene are found only in the oils extracted by hydrodistillation. The chromatograms of the 2 samples and a mixture of hydrocarbons, used to calculate the kovats indexes are shown in figures 1, 2 and 3. The results are summarized in the tables 1 and 2.
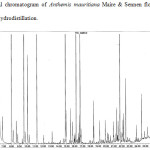 |
Figure 1: Typical chromatogram of Anthemis mauritiana Maire & Sennen flowers essential oil obtained by hydrodistillation. |
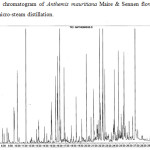 |
Figure 2: Typical chromatogram of Anthemis mauritiana Maire & Sennen flowers essential oil extracted by micro-steam distillation. |
 |
Figure 3: Typical chromatogram of hydrocarbures mixture. |
Table 1: Phytochemical composition (%) of essential oil obtained by hydrodistillation from flowers of Anthemis mauritiana Maire & Sennen.
| Pics | Retention time (min) | Composition (%) | Compounds | KI* |
| 1 | 4.21 | 28,14 | α-Pinene | 1020,88 |
| 2 | 5.29 | 0,11 | Camphene | 1063,07 |
| 3 | 6.47 | 15,57 | β-Pinene | 1100,00 |
| 4 | 6.85 | 0,94 | Sabinene | 1118,76 |
| 5 | 7.99 | 4,75 | β-Myrcene | 1169,38 |
| 6 | 8.21 | 0,38 | α-Terpinene | 1178,71 |
| 7 | 8.61 | 0,59 | Limonene | 1193,95 |
| 8 | 9.57 | 4,28 | γ-Terpinene | 1246,61 |
| 9 | 9.74 | 0,3 | Trans β-Ocimene | 1255,89 |
| 10 | 10.02 | 2,23 | p-Cymene | 1270,83 |
| 11 | 10.24 | 0,23 | α-Terpinolene | 1282,28 |
| 12 | 11.21 | 8,27 | Heptenone-6-Methyl | 1341,40 |
| 13 | 12.06 | 0,16 | Nonyl Aldehyde | 1394,59 |
| 14 | 13.15 | 0,25 | Δ-Elemene | 1471,83 |
| 15 | 14.2 | 0,07 | Nonenol | 1550,75 |
| 16 | 14.84 | 3,64 | β-Caryophylene | 1600,00 |
| 17 | 14.93 | 0,63 | Lavandulyl Acetate | 1607,78 |
| 18 | 15.48 | 0,65 | Sabinyl Acetate | 1654,30 |
| 19 | 15.73 | 0,77 | α-Humulene | 1674,90 |
| 20 | 15.79 | 0,19 | Lavandulol | 1679,80 |
| 21 | 15.96 | 0,09 | α-Copaene | 1693,57 |
| 22 | 16.01 | 0,16 | α-Terpineol | 1697,59 |
| 23 | 16.07 | 0,28 | Cis-Sabinol | 1702,72 |
| 24 | 16.20 | 2,84 | D-Germacrene | 1713,56 |
| 25 | 16.35 | 0,11 | trans-α-Bergamolene | 1727,88 |
| 26 | 16.48 | 0,23 | Bicyclo-Germacrene | 1739,41 |
| 27 | 16.56 | 0,14 | Geranyl Acetate | 1746,47 |
| 28 | 16.61 | 0,5 | Farnesene | 1750,86 |
| 29 | 16.70 | 2,48 | Linalyl-isovalerate | 1757,86 |
| 30 | 16.75 | 0,15 | β-Cadinene | 1763,08 |
| 31 | 16.83 | 0,28 | Lynalyl methylbutanouate | 1770,02 |
| 32 | 18.41 | 0,8 | Phenol | 1900,00 |
| 33 | 18.45 | 0,28 | Neryl Acetate | 1904,55 |
| 34 | 19.2 | 0,48 | oxid Caryophylene | 1988,03 |
| 35 | 20.53 | 0,19 | Spathulenol | 2125,70 |
| 36 | 20.94 | 0,56 | Nonanoic acid | 2168,91 |
| 37 | 20.98 | 0,11 | Δ-Cadinene | 2172,04 |
| 38 | 21.35 | 0,71 | α-Longipinene | 2211,20 |
| 39 | 21.54 | 0,41 | β-Eudesmol | 2233,43 |
| 40 | 21.76 | 5,76 | α-Curcumene | 2257,66 |
| 41 | 21.91 | 0,28 | Decanoic acid | 2274,03 |
| 42 | 22.15 | 0,53 | Tricosane | 2300,00 |
| 43 | 23.88 | 0,33 | Pentacosane | 2500,00 |
| 44 | 25.25 | 0,3 | Heptacosane | 2700,00 |
| 45 | 26.86 | 0,69 | Hexadecanoic acid | 2908,22 |
| Total | 90,84 |
Table 2: Phytochemical Composition of essential oil obtained by micro-steam distillation from flowers of Anthemis mauritiana Maire & Sennen.
| Pics | Retention time (min) | Composition (%) | Compounds | KI* |
| 1 | 4,24 | 15,36 | α-Pinene | 1021,75 |
| 2 | 4,31 | 0,59 | α-phellandrene | 1024,78 |
| 3 | 6,23 | 7,81 | β-Pinene | 1093,00 |
| 4 | 6,61 | 0,2 | Sabinene | 1107,04 |
| 5 | 7,86 | 0,34 | β-Myrcene | 1163,98 |
| 6 | 8,71 | 0,29 | Cineole | 1197,74 |
| 7 | 9,53 | 0,78 | γ-Terpinene | 1243,85 |
| 8 | 10,02 | 2,19 | p-Cymene | 1270,31 |
| 9 | 10,23 | 0,18 | α-Terpinolene | 1281,25 |
| 10 | 11,26 | 9,22 | Methyl heptenone | 1343,99 |
| 11 | 12,72 | 0,27 | α-Thujene | 1441,32 |
| 12 | 12,96 | 0,15 | Acetic acid | 1458,47 |
| 13 | 13,04 | 0,47 | Heptenol methyl | 1464,12 |
| 14 | 13,07 | 0,28 | furancarboxaldehyde | 1466,23 |
| 15 | 13,76 | 0,1 | Camphor | 1516,11 |
| 16 | 13,87 | 0,32 | Benzaldehyde | 1524,87 |
| 17 | 14,19 | 0,25 | Nonenol | 1549,97 |
| 18 | 14,77 | 0,18 | Methyl heptadienone | 1594,06 |
| 19 | 14,84 | 0,72 | β-Caryophylene | 1599,26 |
| 20 | 15,48 | 1,23 | Sabinyl acetate | 1653,47 |
| 21 | 15,79 | 2,31 | Lavandulol | 1678,98 |
| 22 | 16,01 | 0,25 | α-Terpineol | 1696,79 |
| 23 | 16,1 | 6,04 | sabinol acetate | 1704,53 |
| 24 | 16,21 | 0,54 | D-Germacrene | 1757,86 |
| 25 | 16,71 | 1,46 | Linalyl isovalerate | 1758,73 |
| 26 | 16,82 | 0,17 | linalyl methylbutanoate | 1768,29 |
| 27 | 17,12 | 0,11 | Myrtenol | 1794,05 |
| 28 | 18,41 | 0,85 | Phenol | 1912,93 |
| 29 | 19,2 | 0,51 | Caryophylene Oxide | 1989,58 |
| 30 | 19,9 | 2,04 | Octanoic acid | 2060,20 |
| 31 | 20,92 | 1,16 | Nonanoic acid | 2165,78 |
| 32 | 20,98 | 0,19 | Δ-Cadinene | 2172,04 |
| 33 | 21,54 | 0,37 | β-eudesmol | 2232,33 |
| 34 | 21,78 | 3,27 | α-Curcumene | 2258,75 |
| 35 | 21,9 | 0,77 | Decanoic acid | 2271,85 |
| 36 | 22,17 | 1,06 | nonadecane | 2301,17 |
| 37 | 23,04 | 0,2 | Tetracosane | 2401,20 |
| 38 | 23,73 | 0,28 | Dodecanoic acid | 2482,61 |
| 39 | 23,82 | 0,4 | Octadecadienoic acid | 2493,06 |
| 40 | 23,9 | 1,89 | Pentacosane | 2501,39 |
| 41 | 24,35 | 0,26 | Octadecatrienoic acid | 2564,73 |
| 42 | 25,02 | 0,25 | Hexadecenolide | 2664,36 |
| 43 | 25,2 | 0,62 | Tetradecanoic acid | 2692,28 |
| 44 | 25,27 | 2,06 | Heptacosane | 2701,41 |
| 45 | 25,46 | 0,3 | β-fenchene | 2729,46 |
| 46 | 25,97 | 0,53 | Octadecanol | 2901,37 |
| 47 | 26,86 | 5,93 | Hexadecanoic acid | 2907,19 |
| 48 | 29,62 | 0,56 | Octadecenoic acid | |
| 49 | 30,46 | 2,25 | Linoleic acid | |
| Total | 77,56 |
- KovatsRI: Kovats Retention Index relative to n-alkanes.
The EOAM exhibit a dose-dependent scavenging activity against DPPH activity. In fact, the essential oil yielded percentage scavenging activities of 9%, 23%, 31% and 37.8% at concentrations of 10, 25, 50 and 100 µg/ml respectively. It is noted that the Butylated Hydroxytoluene (BHT) used as a known synthetic antioxidant has more efficient scavenging activity than the EOAM with 56.59 ± 0.43 at 100 µg/ml (Table 3).
Table 3: Scavenging effect of Anthemis mauritiana Maire and Sennen flowers essential oil and BHT against DPPH radical.
| 10 µg/ml | 25 µg/ml | 50 µg/ml | 100 µg/ml | |
| AMEO (% inhibition) | 9,04 ± 0,15 | 23,28 ± 0,24 | 31,50 ± 0,15 | 37,80 ± 0,24 |
| BHT (% inhibition) | 47,19±2,24 | 43,30 ± 0,43 | 44,71 ± 0,99 | 56,59 ± 0,43 |
Values are expressed as mean ± SEM of triplicates assays.
Discussion and conclusion
The analysis of the essential oil obtained from flowers of Anthemis mauritiana by hydrodistillation allowed 45compounds to be identified representing,91% of the total oil, while by micro-steam distillation, 49 compounds were identified representing 78% of the oil.
It has been reported that the chemical composition of essential oils varies according to the climatic, seasonal, geographical, and geological conditions that reigns where the plant is collected. (13, 14, 15). Previous studies by GC-MS have shown that the composition of the essential oils of Anthemis differs from one species to another and also depends of the collection year (3).
Free radicals are the most common initiators of oxidative reactions (16). When these radicals react with unsaturated fatty acids, the chain reaction of lipid oxidation starts and become a risk factor for development of many diseases especially atherosclerosis and related arterial complications (17). Indeed, free radicals oxidize the LDL-cholesterol which is converted to oxidized LDL (ox-LDL) having a very atherogenic action (18). Reactive radicals also cause a deleterious action to the skin structure (19). Natural antioxidants can scavenge and react with free radicals, and hence terminate the free radical reaction. The suppression of the oxidative effect of free radicals by anti-oxidants constitute one of the major targets of many therapeutic agents and the preferable strategy to prevent the cardiovascular diseases (20; 21) and skin damages. Essential oil may help to provide protection against oxidative stress by contributing, along with antioxidant vitamins and enzymes, to the total antioxidant defense system of the human body. In fact, many experimental investigations have demonstrated that a number of essential oils isolated from medicinal and aromatic plants possess a high anti-oxidant potential due to their radical scavenging activity and protect very efficiently against some cardiovascular and degenerative diseases (22, 23). Our experimental study has demonstrated that EOAM has an antioxidant power which may contribute to the potential capability of its compounds, added to foods or given therapeutically, to prevent oxidative stress and related events. Many investigators have proposed some mechanisms to explain the antioxidant activity of the natural antioxidants. Firstly, these compounds may directly scavenge free radicals and consequently break chain reaction of lipid peroxidation (12, 9). Secondly, they may also chelate pro-oxidant metal ions such as iron and copper that stimulate free radical formation (12, 9, 24).
α-pinene and β-pinene are the major components of the studied oil. These compounds can be responsible for the radical scavenging effect observed. Our result is in accordance with the work of Wang W (25) demonstrating that these components exhibited remarkable antioxidant activity in the DPPH test system. On the other hand, according to Bektas Tepe (26), γ-terpinene showed important antioxidant activity. However, the anti-radical effect of our oil can be also attributed to other minor compounds which can react individually or in synergy with major compounds. The radical scavenging effect of the studied oil can play a pivotal role in the prevention of oxidative stress and related diseases.
In conclusion, our results showed that the EOAM contains two major compounds, the α-pinene and β-pinene which are very probably responsible for the observed radical scavenging effect. More experimental researches must be designed to confirm this hypothesis. EOAM exhibits antioxidant activity and can be used as alternative medicine to prevent or treat oxidative stress and related complications.
Acknowledgments
Katherine Nott (Belgium), Mustapha Badraoui and Karim Ramdaoui (Morocco) are acknowledged for technical support. This work was supported by a grant from “Programme P3 de la Coopération Universitaire Mohammed Premier-Commission Universitaire de Développemnt (CUD), Belgium”
References
- Oberprieler C. Phylogenetic relationships in Anthemis L. (Compositae, Anthemideae) based on nrDNA ITS sequence variation. Taxon; 50: 745-762 (2001).
- Williams CA, Greenham J, Harborne JB. Biochem Sys Ecol, 29, 929 (2001).
- Saroglou V, Dorizas N, Kypriotakis Z, Skaltsa HD. J. CHROMATOGR.A 1104, 313 (2006).
- Papaioannou P, Lazari D, Karioti A, Souleles C, Heilmann J, Hadjipavlou-Litina D, Skaltsa H. J. Biosci. 62, 326 (2007).
- El Hanbali F, Mellouki F, Akssira M, Boira H, Blázquez MA. J. Essent. Oil Bear. Plants. 10, 499 (2007).
- Maschi O, Dal Cero E, Galli GV, Caruso D, Bosisio E. Dell Agli M. J. Agric. Food Chem. 56, 5015 (2008).
- Karim A, Breaba M, Mekhfi H, Ziyyat A, Legssyer A, Bouali A, Haloui B, Amrani S, Aziz M, J. Smooth Muscle Res. 46, 65 (2010).
- Liao L, Starzyk RM, Granger DN. Arterioscler. Thromb. Vasc. Biol. 17, 437 (1997).
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Am. J. Med.; 113, 71S (2002).
- Wolin MS. Arterioscler. Thromb. Vasc. Biol. 20, 1430 (2000).
- Trichopolou A, Vasilopoulou E. Br. J. Nutr. 84, 205 (2000).
- Fuhrman B, Aviram M. Curr. Opin. Lipidol. 12, 41 (2001).
- Benjilali, B., Sarris, J., & Richard, H. Sci. Aliments, 2, 515 (1982).
- Ouyahya, A., Negre, R., Viano, J., Lozano, Y.F., & Gaydou, E.M. Lebensmittel-Wissenschaft und-Technologie, 23, 528 (1990).
- Lawrence, B.M. Armoise oil. Natural Flavor and Fragrance Materials. In: Perfumer and Flavorist (Ed.), Essential Oils 1988–1991. pp 52–54 (1993).
- Freidovich I.Ann.NY Acad. Sci. 893, 13 (1999).
- Bouhamidi R, Prevost V, Nouvelot A, Plant Biology and Pathalogy: Comptes Rendus de l’Acad´emie des Sciences – Series III – Sciences de la Vie 321, 31 (1998).
- Steinberg, D. and Lewis, A. Circulation, 95, 1062 (1997).
- Jurkiewics BA, Bissett DL, Buettner GR. J. Invest. Dermatol. 104, 484 (1995).
- Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Buring JE. Am. J. Clin. Nutr. 72, 922 (2000).
- Sesso HD, Gaziano JM, Liu S, Buring JE. Am. J. Clin. Nutr. 77, 1400 (2003).
- Erkan N, Ayranci G, Ayranci E. Food Chem.110, 76 (2008).
- Kelen M, Tepe B. Bioresource Technol. 99, 4096 (2008).
- Kris-Etherton PM, Lefevre M, Beecher GR, Gross MD, Keen CL, Etherton TD. Am. J. Clin. Nutr. 24, 511 (2004).
- Wang W, Wu N, Zu YG, Fu YJ. Food Chem. 108, 1019(2008).
- Tepe B, Tepe AS, Daferera D, Polissiou M, Sokmen A. Food Chem. 103, 766 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









