TLC Densitometric Method for the Estimation of Piperine in Ayurvedic Formulation Trikatu Churna
A.Vyas, V. Jain, D. Singh, M. Singh, S. S. Shukla, R. Pandey, Saraf Swarnlata and S. Saraf
University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur - 492 010 (India).
The present study was to develop the fingerprint method for Trikatu churna by simple high-performance thin layer chromatography (HPTLC) determination using piperine as a standard, which is as an important and major content in formulation. HPTLC methods for determination of piperine from the Trikatu churna along with its raw materials have been developed. The method was validated for linearity, accuracy, limit of detection, limit of quantification, inter-day and intra - day assay precision, repeatability of measurement, and repeatability of sample application. The concentration of piperine present in raw materials was found to be 4.2%± 0.43w/w in Piper nigrum (Marica), and 2.15% ± 0.68w/w in Piper longum (Pipali) respectively and in three identical laboratory batch of Trikatu churna name TK-I, TK-II, TK-III, was 2.13%±0.62, 2.42%±0.67, 2.18%±0.41 w/w respectively with mean value 2.24%±0.48 w/w. The piperine content of all the three batches is found to be in close proximities with each other. Obtained results were compared with marketed formulations.
KEYWORDS:Piperine; Trikutu Churna; Ayurvedic Formulation; Fingerprint; Validation; HPTLC; Quality Control
Download this article as:| Copy the following to cite this article: Vyas A, Jain V, Singh D, Singh M, Shukla S. S, Pandey R, Swarnlata S, Saraf S. TLC Densitometric Method for the Estimation of Piperine in Ayurvedic Formulation Trikatu Churna. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Vyas A, Jain V, Singh D, Singh M, Shukla S. S, Pandey R, Swarnlata S, Saraf S. TLC Densitometric Method for the Estimation of Piperine in Ayurvedic Formulation Trikatu Churna. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24861 |
Introduction
Trikatu Churna is well known Ayurvedic Formulation, comprised of the fruits of two medicinal important plants of Piper longum (Pipali) along with Piper nigrum (Marica) and rhizomes of Zingiber officianalis (Saunth). Trikatu churna is a digestive tonic for the assimilation of other foods in the body. It is a rejuvenator and stimulant and regulates kapha and vata. Thus plays an important role in treatment of variety of conditions (1, 2, 3).
The World Health Organization (WHO) has emphasized the need to ensure the quality of medicinal plant products by using modern controlled technique and applying suitable standards (4, 5). For standardization of natural product drugs, single chemical entities, “marker compounds,” may be used as potency standards in high performance thin layer chromatography (HPTLC) analysis (6). HPTLC analysis for marker compounds may provide additional information in the form of “chromatographic fingerprints (7). The present paper is an effort to develop the quality control parameter by the HPTLC chromatographic fingerprint method of Trikatu Churna using piperine as a standard, which is as an important and major content in formulation.
Experimental
Plants
Dried fruits of Piper longum, Piper nigrum, and rhizomes of Zingiber officianalis were purchased from local market Raipur (C.G.) 492010, INDIA and identified morphologically and microscopically and compared with standard Pharmacopoeial Monograph. The sample of crude drug was authenticated by Dept. of Botany, Dr. H. S. Gour Vishwavidyalaya, Sagar (M. P.) INDIA.
Preparation of Trikatu churna
Trikatu Churna, three batch name TK-I, TK-II, TK-III, were prepared in laboratory using method described in Ayurvedic Formulary of India (1978).2
Preparation of standard solution
Standard piperine (98%) was procured from Lancaster England., with the certificate of analysis. A standard solution of piperine was prepared with accurately weighed 1mg into a 10 ml volumetric flask. The content was dissolved in methanol, and volume was made up to 10 ml.
Sample preparation
The piperine extract of Trikatu churna were prepared by refluxing the powdered Trikatu Churna (1gm) with 60 ml methanol for 1 hour. Filter the extract and re reflux the marc left with 40 ml of methanol for another 1hours. Filter and combine the filtrate. Concentrate the methanol extract under vacuum till the semisolid mass is obtained. Dissolve the residue in 75 ml methanol and filter through sintered glass funnel (G-2) by vacuum filtration assembly. The same procedure was performed for each batch of Trikatu Churna, two marketed formulation M1 and M2 and separately powdered Piper longum (Pippali) and Piper nigrum (Marica), and solution (100 ml) of their piperine extract were prepared.
Chromatographic conditions
The instrument used for the estimation, was Camag Linomat V semi automatic sample applicator, Camag TLC scanner 3, CATS V.4.06 software for interpretation of the data, Hamilton syringe and Camag twin trough chamber. The pre coated silica gel G 60 F 254 was used as stationary phase, obtained from E. Merck. The piperine were well resolved on the precoated silica gel G 60 F 254 on aluminum sheets, the mobile phase was toluene: ethyl acetate (70:30v/v), chamber saturation time 20 min, migration distance 70 mm, wavelength scanning at 336 nm, band width 8 mm, slit dimension 5 * 0.45 mm, scanning speed 20 nm/sec, and the source of radiation was a deuterium lamp. All the solvents used were of AR grade, obtained form S. D. Fine Chemicals Ltd., Mumbai. Marketed formulation of Trikatu churna were purchased from a local pharmacy store.
Method Validation
The method was validated for linearity, accuracy, limit of detection, limit of quantification, inter-day and intra – day assay precision, repeatability of measurement, and repeatability of sample application.
Results and Discussion
The TLC procedure was optimized with a view to develop a stability indicating assay method. The standard and the sample were run in different solvent systems. Better results were obtained with mobile phase consisting of toluene: ethyl acetate (70:30v/v), gave Rf values of 0.42±0.03 for piperine [Fig. – 1]. The spot was resolved on the chromatogram that showed the good resolution. To a pre-washed and activated TLC plate, 1-8 µl of standard stock solution of piperine was spotted with Linomat V semi sample applicator. The plate was developed and scanned. The peak areas of each standard were obtained from the software, and a calibration graph of concentration against peak area was plotted. A good linear relationship was obtained over a concentration range of 100-800 ng/spot of piperine. The correlation coefficient (r2) value was 0.9995, indicates the good linearity between the concentration and peak area.
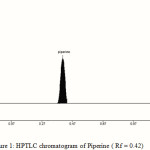 |
Figure 1: HPTLC chromatogram of Piperine ( Rf = 0.42) |
The limit of detection for piperine and the limit of quantification was found to be 100 ng and 0.339μg/ml respectively. These values are considered to be good enough for a reasonable accuracy in most of the laboratories worldwide.
Intra-day assay precision was found by analysis of standard drug at three times on the same day. Inter-day assay precision was carried out using the standard drug at three different days, and % relative standard deviation (RSD) was calculated. The RSD was found to be less than 2 for both inter-day and intra-day assay precision. Repeatability of sample application was assessed by spotting 10 ml of drug solution, 6 times. From the peak areas, the % RSD (0.8420) was determined. Repeatability of measurement was determined by spotting 10 ml of standard drug solution on TLC plate, after development spot was scanned six times without changing position. The % RSD calculated for piperine is 0.3672.
The efficiency of the method is determined by means of number of theoretical plates. It was calculated using the formula, n=16x 2/y 2, where x=Rf value of drugs and y=width of peaks. The number of theoretical plates was found to be 5745. The complete validation parameters are shown in. [Table – 1]
Table: 1 Validation Parameters
| Parameter | Value |
| Rf | 0.42±0.03 |
| Linearity (ng/spot) | 100-800 ng |
| Correlation coeificients r2 | 0.9995 |
| LOD (ng /spot) | 100ng |
| LOQ (μg /spot) | 0.339µg |
| Precision ( %RSD)a) Inter day
b) Intra day |
0.60
0.49 |
| Recovery Studiesa) Accuracy( %RSD)
b) SE c) Recovery% |
0.353
0.400 99.38 |
| Repeatability of sample application | 0.8420 |
| Repeatability of measurements | 0.3672 |
| No. of theoretical plates | 5745 |
Rf : Retention factor, RSD : Relative standard deviation, LOD : Limit of detection, LOQ: Limit of quantification, SE: Standard error
The sample aliquot of raw materials along with laboratory and marketed formulation was applied, and the plate developed with the mobile phase. The band of piperine in sample extract was confirmed by overlaying their UV absorption spectra with those of standard piperine using a Camag TLC scanner 3(Fig. 2).The amount of piperine present in the raw materials and formulations was calculated using the respective calibration graph. As piperine is the chief constituent of the Trikatu Churna and two of its component of fruits of Piper longum (Pippali), Piper nigrum (Marica). The concentration of piperine present in raw material was found to be 4.2%± 0.43w/w in marica, and 2.15% ± 0.68w/w in pippali respectively which is within the reported range of piperine in the crude drugs. By considering the reported yield of the piperine in crude drugs the required piperine content in the formulation of Trikatu churna is within the 2 to 3.4%. The piperine content in three identical laboratory batch of Trikatu churna name TK-I, TK-II, TK-III, was estimated to be 2.13%±0.62, 2.42±0.67, 2.18±0.41w/w respectively with mean value 2.24%±0.48 w/w and its two marketed formulation M1 and M2 are 1.94%±0.78 and 2.02%±0.69 respectively by using the developed methods, which was under the required range except little deviation in marketed formulation.
The recovery studies were carried out for the accuracy parameter. The study was carried out at three levels. To the powdered formulation, the standard drugs of piperine were added at 50% 100% and 150% levels; dilutions were made, and analyzed by the method. The mean of % recovery % RSD and standard error (SE) of three level were calculated, and found to be within the limit, as listed in [Table – 1].
Conclusion
The HPTLC method developed is simple, rapid, precise and accurate.The statistical analysis proved that the method is reproducible and efficient for the analysis of piperine, in Ayurvedic formulations. As Trikatu Churna is a good source of piperine, these findings can be used as routine chromatographic fingerprinting method for the standardization of the Trikatu churna as well as raw materials.
Acknowledgement
The authors are highly grateful to UGC [F. No: 34-131/2008 (SR)] for providing financial assistance under major research project.
References
- Bhaisajyaratnavali, Edn. 2nd, Chowkhamba Sanskrit Series office, Varanasi, 1961: sloke 19, 35.
- Govt. of India, The Ayurvedic Formulary of India, Part I, edn. 1st, Ministry of Health and Family Planning, Dept. of Health, Delhi, xxvii-xxvii: 110 (1978).
- Kumar S.V.K. and Mishra M.S., Indian J Pharm Sci., vol. 66(3), 365-367 (2004).
- WHO, Quality control methods for medicinal plant materials, Geneva. 1-15 (1998a).
- Jain V., Saraf, S. and Saraf, S., Asian J. Chem., vol. 19(2), 1406-1410 (2007).
- Shukla S. S., Saraf, S. and Saraf, S., J. Res. Educ. Indian Med., Vol. XV: 1 (Jan.-March) 2009 page 25-32.
- LazarowychN.J. and Pekos, P., Drug Information Journal, Vol. 32, 497–512 (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









