Synthesis of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3´,2´:2,3][1,2,4] triazolo[1,5-a]pyridine-9-carbonitrile
Maddila Suresh1*, Sreekanth B.Jonnalagadda2 and Chunduri Venkata Rao
1Department of Chemistry, S. V. University, Tirupati - 517 502 (India).
2School of Chemistry, University of KwaZulu-Natal, Westville Campus, (South Africa).
A series of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3',2':2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile derivatives (4a-j) were obtained by the initial reaction of 5-oxo-7-phenyl-2-thiol-3,5-dihydro[1,2,4]-triazolo[1,5-a]pyridine-6,8-dicarbonitrile (3) with chloroacetic acid and appropriate aromatic aldehydes followed by fused sodium acetate condensation. The structures of the newly synthesized compounds were confirmed by IR, 1H NMR, Mass and analytical data. Compounds 4a, 4f, 4i and 4j exhibited good antimicrobial activity.
KEYWORDS:Fused sodium acetate; triazolos; antibacterial; antifungal activities
Download this article as:| Copy the following to cite this article: Suresh M, Jonnalagadda S. B, Rao C. V. Synthesis of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3´,2´:2,3][1,2,4] triazolo[1,5-a]pyridine-9-carbonitrile. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Suresh M, Jonnalagadda S. B, Rao C. V. Synthesis of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3´,2´:2,3][1,2,4] triazolo[1,5-a]pyridine-9-carbonitrile. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24774 |
Introduction
Triazoles are important classes of heterocyclic compounds. In particular, fused 1,2,4-triazoles express antifungal1, bactericidal1,2, anxiolytic3,4, anticonvulsant5 or herbicidal6 activities and can act as antidepressants7. Therefore, versatile and widely applicable methods for their synthesis are of considerable interest. Most methods for the preparation of fused 1,2,4-triazoles are mainly based on hydrazones as precursors. However, these methods have some restrictions regarding their applicability and the use
of toxic reagents like lead tetracetate8,9 and bromine9,10, also the products were formed in low yield and isolated as salts11,12. Many 1,2,4-triazine derivatives are well known to possess biological activities, thus they have found use as herbicides13,14. In the last decade they have been screened in vitro supporting their anti-HIV and anti-cancer activities15-18. However the aza-Wittig reaction is a powerful tool for the synthesis of five- to seven-membered nitrogen heterocycles19-26. Annulation of ring systems with N-heterocycles by means of an aza-Wittig reaction has recently been widely utilized because of the availability of functionalized iminophosphoranes27-31. Many important fused nitrogen
heterocycles such as indole, pyridine, pyrimidine and isoquinoline derivatives have been synthesized via the intramolecular aza-Wittig reaction19-22, as well as by the intermolecular aza-Wittig reaction followed by electrocyclization, intramolecular cycloaddition or heterocyclization23-26. We have previously published the synthesis of fused pyrimidines based on the tandem aza-Wittig annulation strategy32, and as a part of our ongoing studies we now describe a novel one-pot synthesis of 1,2,4-triazolo[1,5-a]-
pyridine and pyrido[1,2-b][1,2,4] triazines derivatives in good yield.
Results and Discussion
The iminophosphorane (2) were synthesized according to the reported method by the reaction with triphenylphosphine/hexachloroethane and triethylamine reagent system (the Appel method, i.e. the modified Kirsanov reaction)33. 5-oxo-7-phenyl-2-thiol-3,5-dihydro[1,2,4]-triazolo[1,5-a]pyridine-6,8-dicarbonitrile (3) was obtained by reaction of iminophosphorane (2) with excess carbon disulfide. Compound 3 on condensation with chloroacetic acid and aromatic aldehydes in boiling acetic acid/acetic anhydride mixture in the presence of fused sodium acetate yielded (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]
pyridine-9-carbonitrile (4a-j) in good yields. The reaction sequences are outlined in Scheme 1.
The IR spectrum of (Z)-2-benzylidene-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H– thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4a) showed an absorption band at 3032 cm-1 indicates the Ar-H stretching. The absorption band at 1730 cm-1 due to the presence of C=O stretching of the thiazolo ring system. Other prominent absorption band is observed at 1590 cm-1 (C=N).
The 300 MHz 1H NMR spectrum of compound 4a showed a singlet at δ 8.20 integrating for one proton, which is attributed to the benzylidene proton. The aromatic protons resonated as three multiplets at δ 7.60-7.55, δ 7.45-7.33 and δ 7.19-7.17.
Further evidence for the formation of compound 4a was obtained by recording its mass spectra. The mass spectrum of compound 4a showed a molecular ion peak at m/z 422 (M+1)+, which is in consistent with its molecular formula C23H11N5O2S. The characterization data of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4a-j) are given in experimental section.
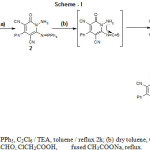 |
Scheme 1: . (a) PPh3, C2Cl6 / TEA, toluene / reflux 2h; (b) dry toluene, CS2;(c) RCHO, ClCH2COOH, fused CH3COONa, reflux. |
| S.No | Compound | S.No | Compound |
| 4a | R=H | 4f | R=Br |
| 4b | R=Cl | 4g | R=OCOCH3 |
| 4c | R=NO2 | 4h | R=CH3 |
| 4d | R=OCH3 | 4i | R=CH(CH3)2 |
| 4e | R=N(CH3)2 | 4j | R=C(CH3)3 |
Experimental
All reagents and solvents were purchased and used without further purification. Melting points were determined on a Fisher–Johns melting point apparatus and are uncorrected. Crude products were purified by column chromatography on silica gel of 60–120 mesh. IR spectra were obtained on a Perkin Elmer BX serried FT-IR 5000 spectrometer using KBr pellet. NMR spectra were recorded on a varian 300 MHz spectrometer for 1H NMR. The chemical shifts were reported as ppm down field using TMS as an internal standard. Mass spectra were recorded on a MASPEC low resolution mass spectrometer operating at 70 eV.
1-amino-6-(triphenylphosphoranylideneamino)-2-oxo-4-phenyl-1,2-dihydro-
pyridine-3,5-dicarbonitrileiminophosphorane (2) were synthesized according to the reported method by the reaction with triphenylphosphine/hexachloroethane and triethylamine reagent system (the Appel method, i.e. the modified Kirsanov reaction)33.
Preparation of 5-oxo-7-phenyl-2-thiol-3,5-dihydro[1,2,4]-triazolo[1,5-a]pyridine-6,8-dicarbonitrile (3).
To a solution of 1-amino-6-(triphenylphosphoranylideneamino)-2-oxo-4-phenyl-1,2-dihydro-pyridine-3,5-dicarbonitrileiminophosphorane 2 (0.5 g, 1.0 mmol) in 15 mL of dry toluene an excess of carbon disulfide (7 mL) was added. The reaction mixture was heated in a sealed tube at 100 °C for 3 h. The crystals that formed were collected and crystallized from a mixture of DMF and H2O (1:1) as yellow crystals.
Synthesis of (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4).
A mixture of 5-oxo-7-phenyl-2-thiol-3,5-dihydro[1,2,4]-triazolo[1,5-a]pyridine-6,8-dicarbonitrile (3) (5 mmol), aromatic aldehydes (5 mmol), chloroacetic acid (5 mmol) and fused sodium acetate (10 mmol) was refluxed in acetic acid/acetic anhydride (25:5 mL) mixture for 3 hours. The reaction mixture was then cooled, filtered and crystallized from acetic acid to give the (Z)-2-(4-substitutedbenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile 4a-j in 66 ~ 89% yields The Rf values were measured using benzene/ethyl acetate mixture as an eluent in ratio (9:1).
(Z)-2-Benzylidene-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H– thiazolo-
[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4a)
White solid in a yield of 87%, mp 146-148 oC; IR (KBr, cm-1): 3032 (Ar-H),
1730 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 8.20 (s, 1H, CH), 7.60-7.55 (m, 2H, Ar-H), 7.45-7.33 (m, 6H, Ar-H), 7.19-7.17 (m, 2H, Ar-H), MS (m/z, %): 422 (M+1)+.Analysis. Calcd for C23H11N5O2S: C, 64.25; H, 2.55; N, 16.57. Found: C, 65.55; H, 2.63; N, 16.62.
(Z)-2-(4-Chlorobenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4b)
White solid in a yield of 66%, mp 171-172 oC; IR (KBr, cm-1): 3030 (Ar-H), 1730 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.68-7.66 (m, 2H, Ar-H), 7.44-7.33 (m, 5H, Ar-H), 7.17-7.15 (m, 2H, Ar-H), MS (m/z, %): 456 (M+1)+. Analysis. Calcd for C23H10ClN5O2S: C, 60.76; H, 2.36; N, 15.52. Found: C, 60.60; H, 2.21; N, 15.36.
(Z)-2-(4-Nitrobenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4c)
Yellow solid in a yield of 79%, mp 201-202 oC; IR (KBr, cm-1): 3050 (Ar-H), 1730 (C=O), 16000 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 8.21-8.20 (m, 2H, Ar-H), 8.03-8.00 (m, 2H, Ar-H), 7.94 (s, 1H, CH), 7.46-7.33 (m, 3H, Ar-H), 7.17-7.15 (m, 2H, Ar-H), MS (m/z, %): 465 (M+1)+. Analysis. Calcd for C23H10N6O4S: C, 59.37; H, 2.29; N, 18.24. Found: C, 59.23; H, 2.16; N, 18.02
(Z)-7-Isocyano-2-(4-methoxybenzylidene)-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4d)
White solid in a yield of 81%, mp 183-184 oC; IR (KBr, cm-1): 3030 (Ar-H), 1710 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.46-7.33 (m, 5H, Ar-H), 6.71-6.70 (m, 2H, Ar-H), 3.06 (s, 3H, OCH3). MS (m/z, %): 452 (M+1)+. Analysis. Calcd for C24H13N5O3S: C, 64.12; H, 2.98; N, 15.06. Found: C, 63.85; H, 2.90; N, 15.51.
(Z)-2-(4-(Dimethylamino)benzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4e)
Yellow solid in a yield of 69%, mp 203-204 oC; IR (KBr, cm-1): 3030 (Ar-H), 1710 (C=O), 1580 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.46-7.33 (m, 5H, Ar-H), 6.71-6.70 (m, 2H, Ar-H), 3.02 (s, 3H, N(CH3)2). MS (m/z, %): 465 (M+1)+. Analysis. Calcd for C25H16N6O2S: C, 64.83; H, 3.56; N, 17.89. Found: C, 64.64; H, 3.47; N, 18.07.
(Z)-2-(4-Bromobenzylidene)-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4f)
White solid in a yield of 89%, mp 194-195 oC; IR (KBr, cm-1): 3030 (Ar-H), 1710 (C=O), 1580 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.68-7.66 (m, 2H, Ar-H), 7.44-7.33 (m, 5H, Ar-H), 7.17-7.15 (m, 2H, Ar-H). MS (m/z, %): 502 (M+2)+. Analysis. Calcd for C23H10BrN5O2S: C, 55.43; H, 2.22; N, 13.88. Found: C, 55.21; H, 2.01; N, 14.00.
(Z)-Methyl 4-((9-cyano-7-isocyano-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridin-2-ylidene)methyl)benzoate (4g)
White solid in a yield of 68%, mp 165-166 oC; IR (KBr, cm-1): 3030 (Ar-H), 1760 (OCO), 1730 (C=O), 1580 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.67-7.66 (m, 2H, CH), 7.49-7.33 (m, 5H, Ar-H), 7.17-7.15 (m, 2H, Ar-H), 3.89 (s, 3H, COCH3). MS (m/z, %): 480 (M+1)+. Analysis. Calcd for C25H13N5O4S: C, 62.89; H, 2.91; N, 14.73. Found: C, 62.63; H, 2.73; N, 14.61.
(Z)-7-Isocyano-2-(4-methylbenzylidene)-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4h)
White solid in a yield of 74%, mp 192-193 oC; IR (KBr, cm-1): 3030 (Ar-H), 1730 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.59-7.57 (m, 2H, Ar-H), 7.40-7.33 (m, 3H, Ar-H), 7.18-7.17 (m, 4H, Ar-H), 2.34 (s, 3H, CH3). MS (m/z, %): 436 (M+1)+. Analysis. Calcd for C24H13N5O2S: C, 66.58; H, 3.18; N, 16.27. Found: C, 66.20; H, 3.01; N, 16.08.
(Z)-7-Isocyano-2-(4-isopropylbenzylidene)-3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4i)
White solid in a yield of 72%, mp 210-211 oC; IR (KBr, cm-1): 3030 (Ar-H), 1730 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.63-7.62 (m, 2H, Ar-H), 7.40-7.25 (m, 5H, Ar-H), 7.17-7.15 (m, 2H, Ar-H), 2.87-2.70 (m, 1H, CH), 1.20 (d, 6H, CH3). MS (m/z, %): 464 (M+1)+. Analysis. Calcd for C26H17N5O2S: C, 67.71; H, 3.88; N, 15.32. Found: C, 67.38; H, 3.70; N, 15.11.
(Z)-2-(4-(Tert-butyl)benzylidene)-7-isocyano–3,6-dioxo-8-phenyl-3,6-dihydro-2H-thiazolo-[3′,2′:2,3][1,2,4]triazolo[1,5-a]pyridine-9-carbonitrile (4j)
White solid in a yield of 75%, mp 201-202 oC; IR (KBr, cm-1): 3030 (Ar-H), 1730 (C=O), 1590 (C=N), 1H NMR (300 MHz, DMSO-d6): δ 7.80 (s, 1H, CH), 7.63-7.62 (m, 2H, Ar-H), 7.40-7.25 (m, 5H, Ar-H), 7.17-7.15 (m, 2H, Ar-H), 1.35 (s, 9H, CH3). MS (m/z, %): 478 (M+1)+. Analysis. Calcd for C27H19N5O2S: C, 68.23; H, 4.17; N, 14.72. Found: C, 67.91; H, 4.01; N, 14.67.
Pharmacological Studies
Antibacterial Activity
The newly synthesized compounds were screened for their antibacterial activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes and Klebsiella pneumoniae (recultured) bacterial strains by disc diffusion method34,35. The investigation of antibacterial screening data revealed that all the tested compounds showed moderate to good bacterial inhibition. The compounds 4a, 4b, 4e, 4f, 4i and 4j showed very good activity against all the bacterial strains.
Antifungal Studies
Newly prepared compounds were screened for their antifungal activity against Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Penicillium marneffei and Trichophyton mentagrophytes (recultured) in DMSO by serial plate dilution method36,37. The antifungal screening data showed moderate to good activity. Compounds 4a, 4c, 4f, 4i and 4j emerged as very active against all the fungal strains.
Pharmacological Assay
Antibacterial Assay
A standard inoculum (1-2 X 107 c.f.u/cm3 0.5 McFarland standards) was introduced on to the surface of sterile agar plates, and a sterile glass spreader was used for even distribution of the inoculum. The discs measuring 6.25 mm in diameter were prepared from Whatman no.1 filter paper and sterilized by dry heat at 140 oC for 1 h. The sterile disc previously soaked in a known concentration of the test compounds were placed in nutrient agar medium. Solvent and growth controls were kept. The plates were inverted and incubated for 24 h at 37 oC. The inhibition zones were measured and compared with the controls. Minimum inhibitory concentration (MIC) was determined by broth dilution technique. The nutrient broth, which contained logarithmic serially two fold diluted amount of test compound and controls were inoculated with approximately
5 X 105 c.f.u of actively dividing bacteria cells. The cultures were incubated for 24 h at 37 oC and the growth was monitored visually and spectrophotometrically. The lowest concentration (highest dilution) required to arrest the growth of bacteria was regarded as minimum inhibitory concentrations (MIC). Ciprofloxacin was used as a standard drug. The diameter of the zone of inhibition and minimum inhibitory concentration values are given in Table 1.
Table 1: Antibacterial activity of thiazolotriazinoes (4a-j).
| Compound no | Staphylococcus aureus | Eschrichia coli | Pseudomonas aeruginosa | Klebsiella pneumoniae | Streptococcus pyogenes |
| 4a | 20 (6.25) | 25 (6.25) | 29 (6.25) | 18 (6.25) | 23 (6.25) |
| 4b | 23 (6.25) | 28 (6.25) | 30 (6.25) | 18 (6.25) | 21 (6.25) |
| 4c | 10 (12.5) | — | — | 17 (6.25) | 11 (6.25) |
| 4d | 8 (25) | 23 (6.25) | 9 (25) | — | 8 (12.5) |
| 4e | 22 (6.25) | 27 (6.25) | 32 (6.25) | 20 (6.25) | 24 (6.25) |
| 4f | 21 (6.25) | 29 (6.25) | 32 (6.25) | 20 (6.25) | 23 (6.25) |
| 4g | 10 (12.5) | 15 (25) | — | — | 17 (12.5) |
| 4h | 12 (12.5) | — | 21 (6.25) | — | 8 (25) |
| 4i | 21 (6.25) | 24 (6.25) | 29 (6.25) | 19 (6.25) | 23 (6.25) |
| 4j | 21 (6.25) | 26 (6.25) | 32 (6.25) | 21 (6.25) | 24 (6.25) |
| Standarda | 24 (6.25) | 30 (6.25) | 33 (6.25) | 23 (6.25) | 25 (6.25) |
— Indicates bacteria is resistant to the compounds at > 100 μg/ml, MIC values are given in brackets. MIC (µg/ml) = minimum inhibitory concentration, ie. Lowest concentration to completely inhibit bacterial growth. Zone of inhibition in mm.
a Ciprofloxacin was used as standard.
Antifungal Assay
Sabourauds agar media was prepared by dissolving 1 g peptone, 4 g D-glucose, and 2 g agar in 100 cm3 distilled water, and adjusting pH to 5.7 using buffer. Normal saline was used to make a suspension of spore of fungal strain for lawning. A loop ful of particular fungal strain was transferred to 3 cm3 saline to get a suspension of corresponding species. 20 cm3 of agar media was poured in to each Petri dish. Excess of suspension was decanted and the plates were dried by placing in a incubator at 37 oC for 1 h. Using an agar punch, wells were made and each well was labeled. A control was also prepared in triplicate and maintained at 37 oC for 3-4 d. The inhibition zones in diameter were measured and compared with the controls. The Nutrient Broth, which contained logarithmic serially two fold diluted amount of test compound and controls was inoculated with approximately 1.6 X 104-6 X 104 c.f.u cm-3. The cultures were incubated for 48 h at 35 oC and the growth was monitored. The lowest concentration (highest dilution) required to arrest the growth of fungus was regarded as minimum inhibitory concentrations (MIC). Amphotericin B was used as the standard drug. The diameter of zone of inhibition and minimum inhibitory concentration values are given in Table 2.
Table 2: Antifungal activity of thiazolotriazinoes (4a-j).
| Compound no | Aspergillus fumigatus | Aspergillus flavus | Trichophyton mentagrophytes | Penicillium marneffei | Candida albicans |
| 4a | 22 (6.25) | 22 (6.25) | 25 (6.25) | 22 (6.25) | 20 (6.25) |
| 4b | 8 (25) | — | 12 (12.5) | — | 17 (6.25) |
| 4c | 22 (6.25) | 20 (6.25) | 22 (6.25) | 25 (6.25) | 17 (6.25) |
| 4d | 15 (6.25) | — | 7 (25) | 21 (6.25) | 18 (6.25) |
| 4e | 5 (25) | 18 (6.25) | — | 12 (12.5) | 17 (6.25) |
| 4f | 24 (6.25) | 21 (6.25) | 21 (6.25) | 23 (6.25) | 18 (6.25) |
| 4g | 11 (12.5) | 12 (25) | — | — | 14 (12.5) |
| 4h | 9 (25) | — | 12 (12.5) | 9 (25) | 10 (12.5) |
| 4i | 22 (6.25) | 19 (6.25) | 20 (6.25) | 23 (6.25) | 19 (6.25) |
| 4j | 21 (6.25) | 26 (6.25) | 32 (6.25) | 21 (6.25) | 24 (6.25) |
| Standardb | 25 (6.25) | 21 (6.25) | 23 (6.25) | 25 (6.25) | 19 (6.25) |
— Indicates fungus is resistant to the compounds at >100 mg/ml, MIC values are given in brackets. MIC (mg/ml) = minimum inhibitory concentration, ie. Lowest concentration to completely inhibit fungal growth. Zone of Inhibition in mm.
b Amphotericin was used as standard.
Conclusion
The investigation of antibacterial screening data reveals that among the 10 compounds screened, four compounds showed good bacterial and fungal inhibition almost equivalent to that of standard.
Acknowledgments
The authors are thankful to the authorities of SriVenkateswaraUniversity, Tirupati, AP (India) for the facilities and encouragement.
References
- El-Hawash S. A., Habib N. S., Fanaki N. H.; Pharmazie., 45, 808-813 (1999).
- Brown D. J., Iwai Y.; Aust. J. Chem., 32, 2727-2733 (1979).
- Tarzia G., Ocelli E., Toja E., Barone D., Corsico N., Gallico L., Luzzani F.; J.Med. Chem., 32, 1115-1123 (1988).
- Trust R. I., Albrigh J. D.; U. S. Patent., 4242515 (1980).
- Tarzia G., Ocelli E., Barone D.; IL Farmaco., 44, 3-16 (1989).
- (a) Peignier R., Cheme A., Cantregril R., Mortier J.; Eur. Patent., 441718.; Chem.Abstr. 115, 208000 (1991).(b) Cantegriil R., Chem A., Mortier J., Peignier R.; Eur. Patent., 483027.; Chem. Abstr. 117, 131214 (1997).
- Sarges S., Howard H. R., Browne R. G., Lbel L. A., Seymour P. A., Koe B. K.; J.Med. Chem., 33, 2240-2254 (1990).
- Bower J. D., Doyle F. P.; J. Chem. Soc., 727-732 (1957).
- Pollak A., Tisler M.; Tetrahedron., 22, 2073-2079 (1966).
- Gibson M. S.; Tetrahedron., 19, 1587-1589 (1963).
- Hadi A., Martin N., Seoane C., Soto J. L.; J. Heterocyclic Chem., 29, 1229(1992).
- Al-Najjar A. A., Amer S. A. R., Riad M., Elghamry I., Elnagdi M. H.; J. Chem.Res (S)., 296-297 (1996).
- Neunhoeffer H.; The Chemistry of Heterocyclic Compounds, (Weissberger A. and Taylor E. C. (eds), 33, 189 (1978). New York, Wiley.
- Neunhoeffer H.; Comprehensive Heterocycl. Chem., (Katritzky A. R. and Rees C.W.(eds), Pargamon Press Oxford 1984, Bolton A. J. and Mckillop A. (Vol. Eds.), 3, Part 2B, 285.
- Abdel-Rahman R. M., Morsy J. M., El-Edfawy S.; Pharmazie., 54, 667 (1999).
- Abdel-Rahman R. M., Seada M., Fawzy M.; Pharmazie., 49, 811 (1994).
- Abdel-Rahman R. M., Morsy J. M., Hanafy F.; Pharmazie., 54, 347 (1999).
- Abdel-Rahman R. M.; Pharmazie., 56, 18 (2001).
- Takeuchi H., Yanagida S., Ozaki T., Hagiwara S., Eguchi S.; J. Org. Chem., 54, 431 (1989).
- Eguchi S., Goto S.; Heterocycl. Commun., 1, 51 (1994).
- Eguchi S., Yamashita K., Matsushita Y.; Synlett., 295 (1992).
- Takeuchi H., Hagiwara S., Eguchi S.; Tetrahedron., 45, 6375 (1989).
- (a) Molina P., Fresneda P. M.; J. Chem. Soc. Chem. Commun., 1819 (1988).(b) Molina P., Alajarin M., Vidal A.; Tetrahedron., 46, 1063 (1990).
- Molina P., Vilaplana M. J.; Synthesis., 474 (1990).]
- (a) Wamhoff H., Schmidt A.; J.Org. Chem., 58, 6976 (1993).(b) Sato T., Ohmori H., Ohkuho T., Motoki S.; J. Chem. Soc. Chem. Commun., 1802 (1993).
- (a) Molina P. M., Alajarin M., Vidal A.; J. Chem. Soc. Chem. Commun., 7 (1990).(b) Molina, P.; Alajarin, M.; Vidal, A.; J. Org. Chem. 55, 6140 (1990).
- Palacios F., Alonso C., Aparicio D., Rubiales G., de los Santos J. M.; Tetrahedron., 63, 523-575 (2007).
- Eguchi S.; Top. Heterocycl. Chem., 6, 113-156 (2006).
- Braese S., Gil C., Knepper K., Zimmermann V. A.; Chem. Int. Ed., 44, 5188 (2005).
- Eguchi S.; Arkivoc., 2, 98-119 (2005).
- Fresneda P. M., Molina P.; Synlett., 1-17 (2004).
- Barsy M. A., El-Rady A. E.; J. Heterocyclic Chem., 43, 523 (2006).
- Appel R., Kleistuk R., Ziehn K. D., Knoll F.; Chem. Ber., 103, 3631 (1970).
- Cruickshank R., Duguid J. P., Marion B. P.; et al. Med. Microbiology, twelveth ed., vol. 2, Churchil Livingstone, London, 196 (1975).
- Collins A. H.; Microbiological Methods, second ed. Butterworth, London, (1976).
- Khan Z. K.; In vitro and vivo screening techniques for bioactivity screening and evaluation, in: Proceeding Int. workshop UNIDO-CDRI 210 (1997).
- Varma R. S.; Antifungal Agents: Past, Present & Future Prospects. NationalAcademy of Chemistry & Biology, India, Lucknow, (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









