Synthesis and Characterization of Hg (II) Complexes with Macrocycic Ligand
Snehika shrivastava1,Vijay Bharti Gupta2,Yogesh Pandey2 and S. C
1I.P.S. College of Technology and Management, Gwalior (India).
2Bipin Bihari P.G. Science College, Jhansi (India).
This paper deals with the synthesis and characterization of a macrocyclic complex compounds of the general formula [M-LX2] where M= Hg(II) resulted from the condensation reaction of diaminopropeane/ diaminobutane and hexanedione with mercury in ethanolic solution. The coordination compounds are formulated according to the chemical analysis, electronic, infrared, 1HNMR and mass spectra, as well as molar conductance values.
KEYWORDS:Metal complexes; Hg(II) metals; Spectroscopy
Download this article as:| Copy the following to cite this article: Shrivastava S, Gupta V. B, Pandey Y, S. C. Synthesis and Characterization of Hg (II) Complexes with Macrocycic Ligand. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Shrivastava S, Gupta V. B, Pandey Y, S. C. Synthesis and Characterization of Hg (II) Complexes with Macrocycic Ligand. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24887 |
Introduction
The coordination chemistry of multidentate macrocycles has been a field of intensive research over the past many years. The tetraazamacrocyclic ligands and their metal complexes have attracted growing interest among coordination chemists followed by many workers on the metal controlled template synthesis of macrocycic species1,2. The chemistry of synthetic macrocyclic polyamines and macrocyclic dioxopolyamines has been drawing much interest3-6(88-91).These macrocycles form much more stable and selective complexes with various transition metal ions than do open chain analogue having the same donor arrangements. These macrocyclic ligands used as models for protein metal binding sites, pigments, vitamin B12, photosynthesis, dioxygen, sodium and potassium ion transport7,8. In biomedical systems9-12 they are used as therapeutic reagents13-15 in chelate therapy for the treatment of metal intoxication, as anti-HIV agents16-18 and as cyclic antibiotic activity is because of specific metal complexation. In the present investigation, the synthesis and structural elucidation of complexes from the reaction of hexannedione with diaminoalkane with Hg(II) metal ions are synthesized and characterized.
Experimental
All solvents were reagent grade and purified as described else where prior to use. HgCl2, Hg(NO3)2, 3,4-hexanedione, diaminopropane and diaminobutane were purchased from Aldrich Chemical and used after purification19. Elemental analyses were made by the microanalytical laboratory obtained from CDRI, Lucknow, India. Metals and chloride were determined volumetrically20 and gravimetrically21 respectively. The IR spectra (4000-200cm-1) of all prepared complexes in CsI pellets were recorded with a Perkin Elmer 621 spectrophotometer. The electrical conductivity of 10-3M solution in DMF was obtained with Digisum Electronic Conductivity Bridge at room temperature. 1H NMR spectra, recorded in DMSO-d6 using a Bruker AC 200E spectrometer with Me4Si as an internal standard, were obtained at the IIT Kanpur, India.
Synthesis of the Tetraazamacrocyclic complexes
To ethanolic solution (20ml) of HgX2 (X=Cl, NO3) (1mmol) a solution of 3,4-hexanedione (2mmol) was added dropwise with constant stirring. This was followed by dropwise addition of 1,3-diaminopropane (2mmol) in ethanol (20ml) with constant stirring for 5hrs. A white solid appeared which was filtered, washed with ethanol and dried under vacuum over CaCl2. [HgL1X2] (Scheme 1).
A similar procedure was adapted for the synthesis of Hg(II) complexes of macrocycles derived from 3,4 hexanedione with 1,4-diaminobutane[HgL2X2].
The complex may be synthesised by the following general method:
HgX2 + 2CH3CH2COCOCH2CH3 + 2H2N(CH2)nNH2 →[HgLX2]
(Where X= Cl or NO3, n=3, 4)
Scheme 1: Synthesis of macrocyclic complexes
Result and Discussion
These synthesised complexes were white solid and stable at room temperature. All the complexes have showed high melting points. Elemental analysis were within ±0.5% for C, H, N, Hg and Cl. The elemental analysis and molar conductance data are represented in Table-1. The molar conductance measurements of the complexes in DMF correspond to non-electrolyte nature[22].
Table 1:Elemental analysis and Molar Conductivity of [HgLX2]
|
S. No. |
Compound |
Found (Calcd) % C H N |
Molar Conductivity ohm-1 cm2 mol-1 (in DMF) |
||
|
1. |
[HgL1Cl2] |
34.2 (34.5) |
5.4 (5.5) |
9.6 (9.7) |
20 |
|
2. |
[HgL2Cl2] |
39.6 (39.7) |
5.6 (5.9) |
9.0 (9.2) |
22 |
|
3. |
[HgL1(NO3)2] |
34.0 (34.1) |
5.0 (5.0) |
13.2 (13.3) |
16 |
|
4. |
[HgL2(NO3)2] |
36.4 (36.5) |
5.2 (5.4) |
12.2 (12.4) |
14 |
The infrared spectra of the metal complexes shown the absence of the stretching modes of functional groups (NH2 and C=O), and the appearance of bands characteristics of imine group[23-25]. The major changes observed in the spectra of the metal complexes are the absence of stretching and deformation vibrations of NH2 group, indicating their deprotonation and the appearance of strong bands due to coordinated √ (C=N) vibrations in the range1626-1605cm-1[23]. Strong and sharp band for C-H bending vibrations appear at ca. 1460-1500 cm-1[23]. The presence of new bands in the spectra of the metal complexes in the region at 4387-457cm-1 due to √(Hg-N) vibration supports the coordination of the imine nitrogen to the mercury ion[26]. In the spectrum of the complexes the bands at cm-1 are observed, which may be assigned to coordinated chloro and nitrate group respectively[23-25].
1H NMR spectrum of one representative Hg(II) complex has been recorded. The α-CH2 protons of the amine residue give a triplet δ-2.63 ppm due to coupling with the β-CH2 protons. The β-CH2 protons of the amine residue give a broad peak at δ-1.50ppm. The remaining methylene protons of the amine residue give rise to a broad peak at δ-1.32 ppm. In macrocyclic precursor, 1,2,8,9-tetraphenyl-3,7-diazaduohepta-2,7-diene-1,9-dione(KIM,3) the α-CH2 protons have been reported to appear as a triplet at δ-3.63ppm and β-CH2 protons as a quintet at δ-2.11ppm[27].
Conclusion
From the reported data, the following chemical structures for the synthesis mixed ligand complexes are proposed.
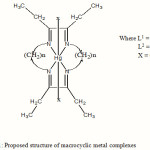 |
Figure 1: Proposed structure of macrocyclic metal complexes Click here to View figure |
Acknowledgement
The authors are highly thankful to Dr. S. N. Shrivastava, Principal, Bipin Bihari P.G. College Jhansi (U.P) for providing necessary facilities to carry out the present research work. We are also grateful to Director C.D.R.I. Lucknow for elemental and analysis and I.I.T Kanpur for spectral studies.
References
- Tadokoro M., Sakiyama H., Matsumoto N., Kodera M., Okawa H. and Kida S., J. Chem. Soc., Dalton Trans., 313 (1992).
- Motoda K.I., Sakiyama H. Matsumoto N., Okawa H. and Kida S., Bull. Chem. Society Japan, 65, 1176 (1992).
- I.M. Kolthoff, Anal. Chem., 51,, 1R-22R (1979).
- K.A. Byriel, V. Gasperov, K. Gloe, C. H.N. Kennard, A.J. Leong, L.F. Lindoy, M.S. Mahinay, H.T. Pham, P.A. Tasker, D. Torp and Turner, J. Chem. Soc. Dalton Trans., 3034 (2003).
- R.R. Fenton, R. Gauci, P.C. Junk, L.F. Lindoy, R.C. Luckay, G.V. Meehan, J.R. Price, P. Turner and G. Wei, J. Chem. Soc. Dalton Trans, 2185 (2002).
- H. Adams, R. Bastida, D.E. Fenton, A. Macias, S.E. Spey and L.V. Valencia, J. Chem. Soc., Dalton Trans., 4131 (1999)
- J.P. Klinman, Chem. Rev., 96,2541 (1996).
- N. Kitajima and Y. Oka-Moro, Chem. Rev., 94,737 (1994).
- R.B. Lauffer, Chem. Rev., 87,901 (1987).
- V.W. Yam and K.K. Lo, Coord. Chem. Rev., 184, 157 (1999).
- B. Barbiier and A. Brack, J.Am.Chem.Soc., 110, 6880 (1998).
- J. Liu, H. Jhang, C. Chen, H. Deng, T. Lu and L. Ji, J. Chem. Soc. Dalton Trans., 114 (2003).
- J.W. Sibert, A.H. Cory and J.G. Cory, Chem. Commun., 154 (2002).
- P.V. Bernhardt and P.C. Sharpe, Inorg. Chem., 39, 4123 (2000).
- V. Alexander, Chem. Rev., 95, 273 (1995).
- L.G. Marzilli, New. J. Chem., 14, 409 (1990).
- D.W. Dixon, R. Schinazi and L.G. Mazilli, Ann. N. Y. Acad. Sci., 616, 511 (1990).
- J.S. Trommel and L.G. Marzilli, Inorg. Chem., 40, 4374 (2001).
- D.D. Perrin and W.L.F. Armergeo, Purification of Laboratory Chemicals, Fourth Edition, The Bath Press, Great Britain (1996).
- C.N.Reilley, R.W. Schmid and F.A. Sadak, J.Chem. Educ. , 36, 555 (1959).
- A.I. Vogel, A Text Book Quantitative Chemical Analysis, Longmans, London
- W.J. Geary, Coord.Chem.Rev.,7,81 (1971).
- L.J. Bellamy, The infrared spectra of complex molecules; Chapman and Hall; London (1978).
- P.K.Rai, R.N. Prasad, Monatsh. Chem., 125, 385 (1994).
- S.M. Nelson, M.Mc. Cann, C. Stevenson, M.G.B. Drew, J.Chem.Soc.Dalton Trans., 1477 (1979).
- M. Sonmez, Polish J.Chem., 77, 397(2003).
- W.A. Welsh, G.J. Reynolds G.J.and P.M. Henry, Inorg.Chem.,16, 2558 (1977).

This work is licensed under a Creative Commons Attribution 4.0 International License.









