Molecular Interactions in Ternary Liquid Mixture of Morpholine, Cyclohexanone and 1-Hexanol at 308.15K and 318.15K
A. Rose Venis1* and X. Rosario Rajkumar2
1PG and Research Department of Chemistry, St. Joseph’s College (Autonomous), Tiruchirappalli - 620 002 (India).
2Oxford Engineering College, Tiruchirappalli - 620 009 (India)
Densities , viscosities and ultrasonic velocities has been measured for the ternary mixture involving morpholine(1) + cyclohexanone(2) + 1- hexanol(3) at 308.15K and 318.15K over the entire range of mole fraction. Parameters like excess volume, adiabatic compressibility, free volume, linear free energy, acoustic impedance, relative association and isentropic compressibility were calculated. From these values the excess properties are also calculated. The deviations of the liquid mixture from ideality have been explained based on the molecular interaction between unlike molecules.
KEYWORDS:Density, Viscosity; Ultrasonic velocity; Adiabatic compressibility; Acoustic impedance
Download this article as:| Copy the following to cite this article: Venis A. R, Rajkumar X. R. Molecular Interactions in Ternary Liquid Mixture of Morpholine, Cyclohexanone and 1-Hexanol at 308.15K and 318.15K. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Venis A. R, Rajkumar X. R. Molecular Interactions in Ternary Liquid Mixture of Morpholine, Cyclohexanone and 1-Hexanol at 308.15K and 318.15K. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24757 |
Introduction
Knowledge of density, viscosity and ultrasonic velocity of liquids and liquid mixtures, both binary1-2 and ternary3-5, is of great importance in predicting the nature of molecular interactions between unlike molecules in industrial process. Various studies have been carried out in the recent past in predicting the nature interactions6-8 through various thermodynamic parameters. A close study of literature shows that only few work have been done using morpholine9-11, a liquid commonly used in petrochemical industries. In the present study the nature of molecular interaction in the ternary liquid mixture involving morpholine (1), cyclohexanone12 (2) and 1-hexanol13 (3) at 308.15K and 318.15K has been carried out.
Materials and method
Morpholine (Merck, Mumbai), cyclohexanone (Merck, Mumbai) and 1- hexanol (Loba chem, Mumbai), all analar grades, were dried using suitable drying agents and distilled based on standard methods14. Ternary liquid mixtures of various compositions were prepared by mixing measured amount of pure liquids in air tight stoppered bottles of 50ml capacity. Densities of pure liquids and liquid mixtures were measured by relative density method using 10ml relative density bottle with an accuracy of ± 0.001kgm-3. Viscosities of all pure and liquid mixtures were measured using Ostwald viscometer of 10ml capacity with an accuracy of ±0.001cP. Ultrasonic velocities of pure and liquid mixtures were measured by a single crystal variable path interferometer (Mittal Enterprises, New Delhi, Model F-80) at a frequency of 2MHz with an accuracy of ±0.02%. All the measurements were made at both 308.15K and 318.15K with the help of a digital thermostat with a temperature accuracy of ± 0.01K.
Results and discussion
The experimental densities (ρ), viscosity (η) and ultrasonic velocity (u) for the pure liquids and ternary system are presented in Table 1, 2(308.15K) and 3(318.15K).
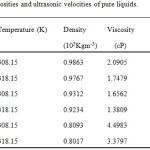 |
Table 1: Densities, viscosities and ultrasonic velocities of pure liquids. Click here to View table |
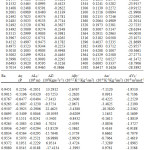 |
Table 2: Densities, viscosities, ultrasonic velocities and acoustic parameters for the ternary mixture at 308.15K. |
Adiabatic compressibility (Ks) has been calculated from Laplace’s equation
Ks = 1/ρu2 (1)
in which ρ and u are density and ultrasonic velocity in liquid mixture.
Acoustic impedance (Z) has been calculated by the relation15
Z= u ρ (2)
Linear free energy has been calculated by Jacobson’s relation16,17
Lf = K/uρ1/2 (3)
K is Jacobson’s constant which is temperature dependent constant but independent of the nature of the liquid.
Viscosity has been calculated using the relation
η = (At – B/t)ρ (4)
A and B are constants characteristic of viscometer calculated using standard liquids water and nitrobenzene, t time of flow.
Surianarayana18 proposed a relation to calculate free volume
Vf = (Meff u/K η)3/2 (5)
K is a temperature independent constant which is equal to 4.28×109 for all liquids; Meff is effective molecular weight of the mixture calculated using the relation
Meff = x1M1+x2M2+x3M3. Where x1, x2, x3, M1, M2, M3 are mole fractions and molar masses of the pure components 1,2 and 3.
Relative association has been calculated using the relation
Ra = (ρ/ρ1)(u1/u)1/3 (6)
Isothermal compressibility has been calculated using the relation
βT = 1.71×10-3/(T4/9u2ρ4/3) (7)
T is absolute temperature
Thermal expansion coefficient has been calculated using the relation
α = (0.0191βT)1/4 (8)
Excess volume (VE) has been calculated using the relation
VE = ((x1M1+x2M2+x3M3)/ρ) – (x1M1/ρ1) –(x2M2/ρ2) –(x3M3/ρ3) (9)
ρ1,ρ2and ρ3 are densities of pure components 1,2 and 3.
Excess adiabatic compressibility (ΔKs) has been calculated from the relation
ΔKs = Ks – (φ1Ks1 + φ2Ks2 +φ3Ks3) (10)
Ks1, Ks2, Ks3 are adiabatic compressibility values of pure liquids and φ1,φ2 and φ3 are volume fraction for pure liquids calculated by the relation
Φ1 =(x1M1/ρ1)/(x1M1/ρ1 + x2M2/ρ2 + x3M3/ρ3) (11)
Excess values of other parameters are calculated using the relation
AE = Aexp – Aid (12)
Aid = ∑ xi Ai , xi and A i are mole fraction and parameters of the ith component liquid.
All the calculated excess values were fitted to Redlich – Kister19 type polynomial equation
AE = x1x2x3[a+bx1(x2-x3) + cx12(x2-x3)2] (13)
by the method of least squares to derive the adjustable parameters a, b and c. From these a, b and c values theoretical values for all excess parameters were calculated and the standard deviation values were calculated using the relation
σ = [(AE exp – AE cal )2/(n-m)]1/2 (14)
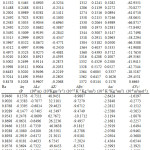 |
Table 3 : Densities, viscosities , ultrasonic velocities and acoustic parameters for the ternary mixture at 318.15K. |
here n is the number of measurements and m the number of adjustable parameters. The values of a, b, c and σ are given in the table 4a and 4b.
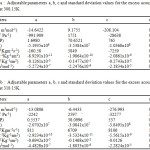 |
Table 4a : Adjustable parameters a, b, c and standard deviation values for the excess acoustical values at 308.15K.Table 4b : Adjustable parameters a, b, c and standard deviation values for the excess acoustical values at 318.15K. Click here to View table |
Excess volume values are negative over entire mole fraction values at 308.15K predicting the presence of strong intermolecular dipolar interaction8,20 between the constituent liquids in the mixture. The interactions may be due to strong hydrogen bonding between 1-hexanol and morpholine, due to electron donor – acceptor complex formation nature of cyclohexanone and geometrical fitting between mixing liquids. Due to this volume contraction takes place. As temperature is raised to 318.15K negative value decreases, except at few mole fractions, that is value become more positive. Since at higher temperatures aggregates of pure liquids open up and move away thus decreasing the excess volume. The above nature of interaction is also predicted by the negative values of adiabatic compressibility and linear free energy values21. As the temperature is increased to 318.15K the adiabatic compressibility values becomes more negative. The positive values of excess viscosity at higher mole fraction of morpholine indicate that flow of this mixture is difficult as compared with pure liquids8. Few values are negative, at lower concentration of morpholine, indicates the easy flow of the mixture at these compositions. Close perusal of the excess values of Z, β, α, Vf, which are all negative, also predicts the presence of strong interaction between morpholine, cyclohexanone and isoamyl alcohol at all mole fractions22-25. The Ra values are positive supporting strong interaction between constituent liquids predominating over hydrogen bonded interaction. At 318.15K all the excess values of Z, β, α, Vf shows a similar trend, but their corresponding values decreases, predicting a decrease in the nature of interaction at higher temperatures.
Conclusion
From density, viscosity and ultrasonic velocity, related acoustical parameters and their excess values for the ternary liquid mixtures of morpholine, cyclohexanone and 1- hexanol for various mole fractions at 308.15K and 318.15K has been studied. It is found that there predominates dipole-dipole type of interaction and donor-acceptor type of complex formation in the liquid mixture.
References
- Rosario Rajkumar X , Raman K.V. and Arulraj S.J., Proc. Indian Acad. Sci., (Chem. Sci),90, 417 (1981)
- Sengwa R.J., Vinita Khatri and Sonu Sankhla, Indian J. Chem., 48A, 512 (2009)
- Thirumaran S. and Earnest Jayakumar, Indian J. Pure & Applied Phys., 47,265 (2009)
- Venkatesu P., Basa Goud B. and Prabhakara Rao M.V., Fluid Phase Equilibria, 120,205(1996)
- Poonam Rathore and Mukhtar Singh, Indian J. Chem., 45A, 2650 (2006)
- Venkatesu P., Thermo Chim. Acta, 443(1), 62 (2006)
- Ali A., J. Phy. & Chem. Liq., 38(4), 459 (2000)
- Sharada Ghosh, Pande K.N. and Wankhade Y.D., Indian J Pure & Appl. Phy., 42, 729 (2004)
- Pettenati C., Alessi P., Fermegila M. and Kikic I., Thermochimica Acta 162, 203 (1990)
- Kozin V. G. and Mukhamadiev A.A., Russian J. Applied Chem., 75 (7), 1061 (2002)
- Akl M. Awwad, J. Chem. Eng. Data 53, 307 (2008)
- Nikos G Tsierkezos, Ioanna E. Meolinou and Alexander C. Filippou, J. Sol. Chem., 34(12), 1371,(2005)
- Vasantharani P., Kalaimagal P. and Kannappan K.N., Asian J. Appl. Sci., 2(1), 96, (2009).
- Furnissv, Hannaford A.J., Smith P.W.G. and Tatchell A.R., “Vogel’s Text book of Practical Organic Chemistry”, 5th ed., chap. 2, Longman, London, 165.
- Nikam P.S. and Hasan M., Indian J. Pure Appl. Phys., 24,502 (1986)
- Jacobson B., Acta Chem. Scand., 6,485 (1952)
- Jacobson B., J. Chem. Phys., 20,927 (1952)
- Suryanarayana C.V., J. Acoust. Soc. India, 7, 107 (1976)
- Redlich O., Kister A.T., Ind. End. Chem., 40,345 (1948)
- Savitha Jyostna T. and Satyanarayana, Indian J. Chem., 44A, 1365 (2005)
- Rama Rao G.V., Viswanatha Sarma A. and Rambabu C., Indian J. Chem., 43A, 2518 (2004)
- Nath G. Sahu and Paikaray, Indian J. Physics, 83 (4), 429 (2009) 429
- Acharya S., Das B.K. and Mohanty G.C., Indian J. Phys. 83 (2), 185 (2009)
- Subba Rao G., Ramajappa T. and Kajagopal E., Indian J. Pure Appl. Phy., 26, 717 (1988)717
- Anwar Ali, Anil Kumar Nain, Vinod Kumar Sharma and Shakil Ahmad, Indian J. Pure Appl.Phy. 42,666 (2004)

This work is licensed under a Creative Commons Attribution 4.0 International License.









