Efficient Ruthenium-Catalyzed Double Condensation of Indoles and Ethereal Dialdehydes
Khalil Tabatabaeian*, Alireza Khorshidi, Tooraj Eftekhari and Shima Khairandish
Department of Chemistry, Faculty of Science, University of Guilan, (Iran)
Article Received on :
Article Accepted on :
Article Published : 05 Mar 2011
RuCl3 .nH2 O was applied as an efficient catalyst for double condensation of indoles and ethereal dialdehydes. It was found that the catalytic system involving RuIII affords bis(indolyl) derivatives under mild reaction conditions in good yields.
KEYWORDS:Ruthenium; Ethereal dialdehydes; Indole; Bis(indolyl)methanes
Download this article as:| Copy the following to cite this article: Tabatabaeian K, Khorshidi A, Eftekhari T, Khairandish S. Efficient Ruthenium-Catalyzed Double Condensation of Indoles and Ethereal Dialdehydes. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Tabatabaeian K, Khorshidi A, Eftekhari T, Khairandish S. Efficient Ruthenium-Catalyzed Double Condensation of Indoles and Ethereal Dialdehydes. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=11649 |
Introduction
Lewis acids play key roles in a large number of reactions, and their use in organic synthesis continues to see rapid development [1]. Transition metal Lewis acid catalysts have emerged as a new class of compounds within this area. They offer neutral and mild conditions that are of interest for the needs of modern chemistry and its focus on economically and ecologically friendly methods. In comparison with classic Lewis acids derived from main group halides (e.g., B, Al, Sn), f-elements, and early transition metal halides, late transition metal Lewis acids often are more inert to ubiquitous impurities such as water, offer higher stability, tunable properties by ligand modification, and a well-defined structure and coordination chemistry, thus allowing detailed studies of reaction mechanisms, and a rational basis for catalyst optimization. Among this new class of late transition metal Lewis acids, ruthenium salts and complexes display remarkable properties [2].
On the other hand, investigation of the chemistry of indoles has been, and continues to be, one of the most active areas of heterocyclic chemistry [3]. Indole derivatives are found abundantly in a variety of natural plants and exhibit various physiological properties and are potentially bioactive compounds. Bis(indolyl)methanes and bis(indolyl)ethanes are important derivatives of indole. Bis(indolyl)methanes (BIMs) are the most active cruciferous substances for promoting beneficial estrogen metabolism in women and men [4]. BIM increase the body’s natural metabolism of hormones and promote good estrogen (2-hydroxyestrogen). This indole antioxidant is patented for alleviating symptoms of fibromyalgia. BIM is effective in the prevention of cancer due to its ability to modulate certain cancer causing estrogen metabolites [5].Scientists have demonstrated that BIM induces apoptosis in human cancer cells and may also normalize abnormal cell growth associated with cervical dysplasia. Thus indole and its derivatives have been a topic of research interest [6].
 |
Scheme 1 |
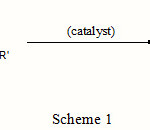
The electrophilic substitution reaction of indoles with aldehydes or ketones afford corresponding bis(indolyl)alkanes (Scheme 1). Numerous methods have been reported for the synthesis of bis(indolyl)methanes. Of these methods, the acid catalyzed electrophilic addition reaction of indole with aldehydes is one of the most simple and straightforward approaches for the synthesis of bis(indolyl)methanes. A variety of reagents such as protic acids (acetic acid) [7], iodine [8,9], clays [10,11],amberlyst-15 [12], In(OTf)3, InCl3 [13], Dy(OTf)3 [14], Ln(OTf)3 [15], FeCl3 [16], ZrCl4 [17], GaX3 [18], alum (KAl(SO4)2.12H2O) [19] and etc. have been employed to promote these reactions. 3-Position of indole is the preferred site for electrophilic substitution reactions and 3-alkyl or acyl indoles are versatile intermediates for the synthesis of a wide range of indole derivatives [20].
Results and Discussion
Recently, we have reported that Ruthenium (III) chloride hydrate is a very effective catalyst for the double addition of indoles to aldehydes or ketones, yielding bis(indolyl) derivatives in high yields and the ruthenium catalyst was used in low concentrations (as low as 1.2 mol% relative to each aldehyde group) [21].
In this contribution, we report the synthesis of new derivatives of bis(indolyl)methanes as potential biologically active compounds. The corresponding derivatives were formed when indoles were treated with various ethereal dialdehydes in the presence of a catalytic amount of RuIII (Scheme 2).
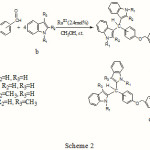 |
Scheme 2 |
Condensation reactions proceeded smoothly at room temperature in rather short times (checked by TLC). Treatment of 1,4-bis(2-formylphenoxymethyle)benzene (0.5 mmol) with indole (2 mmol) in the presence of RuCl3.nH2O catalyst (2.4 mol %) in methanol (6 ml) at room temperature for 30 min. gave corresponding product (1c) in 72% yield (Table 1, entry 1).
These results are summarized in Table 1, and clearly reveal the scope of the reaction.
 |
Table 1: RuIII-catalyzed condensation of indoles and dialdehydes Click here to View table |
Effect of the reaction solvent was monitored by using three different solvents. Methanol was found as the best solvent (Table 2).
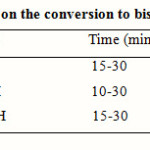 |
Table 2: Effect of solvent on the conversion to bis(indolyl)methanes c, d Click here to View table |
The unique feature of the reaction is that all products were insoluble in the reaction solvent and this made the workup even easier (see experimental).
With regard to regioselectivity, as it is evident from 1H NMR data, preferred site for electrophilic substitution was found to be C-3 position of indole. In the case of 3-methylindole, the reaction proceeded smoothly at C-2 position. Formation of azafulvenium salt which undergoes further addition with a second indole, rationalizes the formation of products from a mechanistic point of view [22].
Conclusion
In summary, a mild, very simple and efficient method has been developed for the synthesis of new indole derivatives as bis(indolyl)methanes via aldol condensation of indoles and various ethereal dialdehydes.
Experimental
General Information
IR spectra were recorded on a Shimadzu FTIR-8400S spectrometer. 1H NMR spectra were obtained on a Bruker (DRX-500 Avance) and 13C NMR spectra were obtained on a Bruker (DRX-125 Avance). Chemical shifts of 1H and 13C NMR spectra were expressed in parts per million downfield from tetramethylsilane. Melting points were measured on a BÜCHI Melting Point B-540 and are uncorrected. Elemental analyses were made by a Carlo-Erba EA1110 CNNO-S analyzer and agreed with the calculated values.
Materials
Chemicals were purchased from Merck and were used without further purification. Ethereal dialdehydes were prepared using the reported procedure [23].
General Procedure for Synthesis of Bis(indolyl)methanes
A mixture of indole (2 mmol), ethereal dialdehyde (0.5 mmol) and RuCl3.nH2O (2.6 mg, 0.012 mmol) in methanol (6 ml) was stirred at room temperature for the appropriate time (Table 1). The progress of the reaction was monitored by TLC. After completion of the reaction, the precipitated product was filtered off and rinsed with cold reaction solvent, which provided the pure product.
Characterization data for the products
1,4-bis{2-[3,3′-bis(indolyl)-methyl]-phenoxy methyl}benzene (1c)
Violet solid; M.P. 176-178 ºC; IR (KBr): υ (cm-1); 742, 1093, 1238, 1454, 1485, 1555, 1593, 2923, 3053, 3413. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 5.01 (s, 2H), 6.39 (s, 1H), 6.50 (d, J = 1.6 Hz, 2H), 6.80-6.90 (t, 1H), 6.91 (s, 2H), 7.00 (t, J = 7.9 Hz, 3H), 7.16-7.18 (m, 4H), 7.20 (d, J = 8.1 Hz, 2H), 7.40 (d, J = 7.8 Hz, 2H), 7.69 (s, 2H, NH) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 37.90, 70.00, 111.60, 116.50, 118.50, 119.30, 121.20, 123.00, 124.20, 124.40, 126.70, 127.70, 128. 80, 133.10, 133.20, 136.90, 138.50, 147.60 ppm. Anal. Calcd. for C54H42N4O2: C, 83.26; H, 5.43; N, 7.19; found: C, 83.23; H, 5.44; N, 7.20.
1,4-bis{2-[3,3′-bis(1-methyl-indolyl)-methyl]-phenoxy methyl}benzene (2c)
Purple solid; M.P. 198-200 ºC; IR (KBr): υ (cm-1); 740, 1101, 1240, 1458, 1480, 1550, 1593, 2925, 3053, 3412. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 3.67 (s, 6H), 5.06 (s, 2H), 5.18 (s, 1H), 6.60 (s, 2H), 6.89 (d, J = 7.96 Hz, 2H), 6.98-7.04 (m, 4H), 7.19-7.26 (m, 5H), 7.29-7.31 (m, 2H), 7.45 (s, 2H) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 12.80, 40.30, 70.20, 111.20, 112.40, 114.20, 118.00, 120.50, 122.30, 123.40, 126.50, 126.80, 127.40, 127.90, 128.20, 130.10, 137.50, 140.10, 157.80 ppm. Anal. Calcd. for C58H50N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.45; H, 6.01; N, 6.71.
1,4-bis{2-[3,3′-bis(2-methyl-indolyl)-methyl]-phenoxy methyl}benzene (3c)
Violet solid; M.P. 221-223 ºC; IR (KBr): υ (cm-1); 742, 1097, 1217, 1456, 1483, 1520, 1589, 2860, 2914, 3024, 3051, 3400. 1H NMR (500 MHz, DMSO-d6, 25 ºC): δ 1.99 (s, 6H) 4.90 (s, 2H), 6.10 (s, 2H), 6.60 (t, J = 7.5 Hz, 2H), 6.81-6.84 (m, 5H), 6.88 (t, J = 7.4 Hz, 2H), 7.00-7.06 (m, 2H), 7.20-7.24 (m, 3H), 10.68 (s, 2H, NH) ppm. 13C NMR (125 MHz, DMSO-d6, 25 ºC): δ 12.20, 40.90, 70.00, 111.60, 112.50, 114.50, 119.30, 121.10, 123.00, 126.70, 128.90, 130.40, 131.20, 136.90, 138.50, 158.80 ppm. Anal. Calcd. for C58H50N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.46; H, 6.02; N, 6.70.
1,4-bis{2-[2,2′-bis(3-methyl-indolyl)-methyl]-phenoxy methyl}benzene (4d)
Purple solid; M.P. 168-169 ºC; IR (KBr): υ (cm-1); 742, 1103, 1240, 1458, 1541, 1615, 2921, 3051, 3412. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 2.30 (s, 6H) 5.10 (s, 2H), 5.40 (s, 1H), 6.62 (d, J = 7.6 Hz, 1H), 6.70 (t, J = 7.6 Hz, 1H), 6.90 (d, J = 7.7 Hz, 1H), 6.95 (t, J = 7.6 Hz, 1H), 7.12 (s, 2H), 7.25-7.31 (m, 8H), 9.98 (s, 2H, NH) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 12.70, 38.20, 71.20, 107.70, 111.10, 114.30, 120.20, 121.30, 122.00, 123.30, 126.80, 127.40, 127.50, 130.10, 130.40, 136.70, 136.90, 140.2, 158.70 ppm. Anal. Calcd. for C58H50N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.40; H, 6.06; N, 6.70.
1,4-bis{4-[3,3′-bis(indolyl)-methyl]-phenoxy methyl}benzene (5c)
Orange solid; M.P. 149-150 ºC; IR (KBr): υ (cm-1); 742, 1091, 1234, 1456, 1506, 1541, 1608, 2923, 3053, 3413. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 5.00 (s, 2H), 5.70 (s, 1H), 6.80 (d, J = 2 Hz, 1H), 6.84-6.87 (t, J = 7.6 Hz, 2H), 6.90 (d, J = 8.6 Hz, 2H), 7.00 (t, J = 7.7 Hz, 2H), 7.20 (t, J = 7.7 Hz, 4H), 7.30 (d, J = 8.1 Hz, 2H), 7.40 (s, 2H), 10.70 (s, 2H, NH) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 55.40, 70.40, 111.20, 112.20 114.10, 118.90, 120.30, 122.20, 122.80, 127.40, 127.50, 130. 20, 130.40, 136.40, 141.10, 157.80 ppm. Anal. Calcd. for C54H42N4O2: C, 83.26; H, 5.43; N, 7.19; found: C, 83.23; H, 5.45; N, 7.21.
1,4-bis{4-[3,3′-bis(1-methyl-indolyl)-methyl]-phenoxy methyl}benzene (6c)
Orange solid; M.P. 212-214 ºC; IR (KBr): υ (cm-1); 740, 1101, 1240, 1458, 1480, 1550, 1593, 2925, 3053, 3412. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 3.60 (s, 6H), 5.19 (s, 2H), 5.42 (s, 1H), 6.58 (s, 2H), 6.89 (d, J = 7.96 Hz, 2H), 6.98-7.04 (m, 4H), 7.19-7.26 (m, 5H), 7.29-7.31 (m, 2H), 7.45 (s, 2H) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 39.70, 40.30, 70.20, 111.20, 112.40, 114.20, 118.00, 120.50, 122.30, 123.40, 126.50, 126.80, 127.40, 127.90, 128.20, 130.10, 137.50, 140.10, 157.80 ppm. Anal. Calcd. for C58H50N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.46; H, 6.01; N, 6.69.
1,4-bis{4-[3,3′-bis(2-methyl-indolyl)-methyl]-phenoxy methyl}benzene (7c)
Red solid; M.P. 241-243 ºC; IR (KBr): υ (cm-1); 744, 1107, 1298, 1377, 1460, 1504, 1540, 1602, 2916, 3055, 3409. 1H NMR (500 MHz, DMSO-d6, 25 ºC): δ 2.00 (s, 6H) 5.00 (s, 2H), 5.80 (s, 1H), 6.60 (t, J = 7.6 Hz, 2H), 6.80 (d, J = 7.9 Hz, 2H), 6.87-6.91 (m, 4H), 7.00 (d, J = 8.5 Hz, 2H), 7.20 (d, J = 7.9 Hz, 2H), 7.40 (s, 2H), 10.71 (s, 2H, NH) ppm. 13C NMR (125 MHz, DMSO-d6, 25 ºC): δ 13.90, 40.10, 70.90, 111.20, 112.80, 114.20, 119.20, 120.10, 122.10, 127.40, 127.80, 130.10, 130.60, 131.50, 136.10, 140.70, 157.80 ppm. Anal. Calcd. for C58H50N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.44; H, 6.03; N, 6.71.
1,4-bis{4-[2,2′-bis(3-methyl-indolyl)-methyl]-phenoxy methyl}benzene (8d)
Red solid; M.P. 142-144 ºC; IR (KBr): υ (cm-1); 743, 1107, 1238, 1454, 1508, 1550, 1593, 2923, 3051, 3413. 1H NMR (500 MHz, CDCl3, 25 ºC): δ 1.99 (s, 6H) 5.20 (s, 2H), 5.50 (s, 1H), 6.55 (d, J = 7.4 Hz, 2H), 6.85 (t, J = 7.4 Hz, 2H), 7.20 (s, 2H), 7.28-7.31 (m, 8H), 10.20 (s, 2H, NH) ppm. 13C NMR (125 MHz, CDCl3, 25 ºC): δ 10.50, 35.20, 70.20, 109.70, 111.10, 113.90, 118.70, 119.90, 122.20, 127.40, 127.80, 131.10, 131.40, 136.20, 136.300, 141.2, 155.80 ppm. Anal. Calcd. for C58H42N4O2: C, 83.42; H, 6.04; N, 6.71; found: C, 83.39; H, 6.07; N, 6.70.
Acknowledgements
Partial support of this study by the research council of University of Guilan is gratefully acknowledged. The authors are also, thankful to Dr. N, Mahmoodi and Dr. H, Kiyani.
References
- Yamamoto H. Wiley-VCH: Weinheim, 2000
- MurahashiSI. Ruthenium In Organic Synthesis; Wiley-VCH: New York, 2004
- Gilchrist T L. Heterocyclic Chemistry; Academic Press, London, 1997
- Li J T, Dai H G, Xu W Z, Li TS. J. Ultrasonics Sonochemistry. 2006, 13: 24
- Michnovicz J J, Bradlow H L, in: Huang M J, Osawa T, Ho C T, Rosen R T (Eds.). American Chemical Society: Washington, D.C.,1994
- Karthik M,Tripathi A K,Gupta N M, Palanichamy M, Murugesan V. Catal. Commun.2004, 5: 371
- Kamal A, Qureshi A A. Tetrahedron 1963, 19: 513
- Bandgar B P, Shaikh K A. Tetrahedron Lett. 2003, 44: 1959
- Ji S J, Wang S Y, Zhang Y, Loh T P. Tetrahedron 2004, 60: 2051
- Yadav J S, Reddy B V S, Satheesh G. Tetrahedron Lett. 2004, 45: 3673
- Chakrabarty M, Gosh N, Basak R, Harigaya Y. Tetrahedron Lett. 2002, 43: 4075
- Farhanullah S A, Maulik P R, Ram V J. Tetrahedron Lett. 2004, 45: 5099
- Nagarajan R, Perumal P T. Tetrahedron. 2002, 58: 1229
- Mi X L, Luo S Z, He J Q, Cheng J P. Tetrahedron Lett. 2004, 45: 4567
- Chen D, Yu L, Wang P G. Tetrahedron Lett. 1996, 37: 4467
- Ji S J, Zhou M F, Gu D G, Jiang Z Q, Loh T P. Eur. J. Org. Chem. 2004: 1584
- Nagawade R N, Shinde D B. Bull. Korean Chem. Soc. 2005, 26: 1962
- Yadav J S, Reddy BVS, Padmavani B, Gupta M K.Tetrahedron Lett. 2004, 45: 7577
- Sonar S S, Sadaphal S A, Kategaonkar A H, Pokalwar R U, Shingate B B, Shingare M S. Bull. Korean Chem. Soc. 2009, 30: 825
- Chakrabarty M, Basak R, Ghosh N, Harigaya Y. Tetrahedron. 2004, 60: 1941
- Tabatabaeian K, Mamaghani M, Mahmoodi N. Khorshidi A. Can. J. Chem. 2006, 84:1541
- Firouzabadi H, Iranpoor N, Jafari A A. J. Mol. Cat. A: Chemical, 2006, 244: 168
- Panda P K, Lee C H. J. Org. Chem. 2005, 70: 3148

This work is licensed under a Creative Commons Attribution 4.0 International License.









