Comparative Kinetic and Mechanistic Study of Oxidation of Benzoic, o-toluic Benzoic, p- Toluic Benzoic acid Hydrazides with Thallium (III) in Acidic Medium
Amit Varale
Department of Chemistry, Changu Kana Thakur Arts, Commerce and Science College, New Panvel District Raigad (India).
The kinetics and Mechanism of oxidation of benzoic, o- Toluic benzoic and p- Toluic benzoic acid hydrazides by thallium(III) in acidic is carried out iodometrically by keeping ionic strength of reaction mixture constant. The reaction proceeds through formation of complex with reactant, which decomposes in subsequent steps to give product. The increase in [H+] and [Cl–] decreases the rate of the reaction. The thermodynamic parameters were also determined and a mechanism is predicted.
KEYWORDS:Kinetics; Thallium(III); Oxidation
Download this article as:| Copy the following to cite this article: Varale A. Comparative Kinetic and Mechanistic Study of Oxidation of Benzoic, o-toluic Benzoic, p- Toluic Benzoic acid Hydrazides with Thallium (III) in Acidic Medium. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Varale A. Comparative Kinetic and Mechanistic Study of Oxidation of Benzoic, o-toluic Benzoic, p- Toluic Benzoic acid Hydrazides with Thallium (III) in Acidic Medium. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24764 |
Introduction
A detail investigation of oxidation of carboxylic acid hydrazide by thallium(III) have not been received much attention. Therefore, with a view to develop a new method, the present study was carried out. The reaction of hydrazides with most oxidants give the corresponding acids1 and in some cases2 esters or amides. The hydrazides are pharmaceutically important compounds used as antitubercular3 and antibacterial4,5 agents, some of them have been reported to possess anti-inflammatory6 and diuretic7 activities. Interest in the use of thallium(III) in the oxidation of organic compounds has increased only recently and research in this regard has not been extensive. The thallium(III) oxidations of several other aliphatic, aryl aliphatic and cyclic ketones have been examined8. The chemical literature has described thallium-induced splitting of carbon-nitrogen bonds9 no mechanistic investigation has been carried out. The present work deals with kinetic and mechanistic study of oxidation of benzoic,o- Toluic benzoic and p- Toluic benzoic acid hydrazide in hydrochloric acid medium.
Experimental
Thallium (III) solution was prepared by dissolving Tl2O3 (ACROS) in 1.0moldm-3 HCl and the concentration was ascertained by iodometric titration. The heterocyclic acid hydrazides were prepared from reported10 procedure and characterized by determining their melting points. Stock solution of benzoic, o- Toluic benzoic and p- Toluic benzoic acid hydrazides were prepared in 50 % v/v, 1,4-dioxan. Ionic strength was kept constant.
The reactions were carried out in 50 % v/v 1-4 dioxane (s.d.fine.chem) under pseudo first order conditions keeping concentration of hydrazide in large excess over that of the oxidant. The solutions containing the reactants and all other constituents were thermally equilibrated separately, mixed and the reaction mixture was analysed for unreacted thallium (III) iodometrically by titrating against standard thiosulphate. The pseudo-first order rate constants were determined from the slopes of linear log[Tl(III)] versus time plots. The results were reproducible up to ± 5 %. Kinetic runs were followed to about three half-lives of the reactions. Under the experimental condition oxidation of 1,4-dioxan did not occur.
End Product Analysis
For identification of products the reaction was carried out by using aqueous solution of hydrazide, Thallium(III), HCl and HClO4. The flask containing reaction mixture was kept in thermostated water bath maintained at 500C for 24 hours to complete the reaction, the residue obtained after filtration was analysed for acid as follows
The presence of carboxylic acid group was detected by testing with bicarbonate.
The formation of acid was confirmed by IR and its melting point.
![]()
Results and Discussion
The reaction occurs rapidly in perchloric acid medium but in the presence of hydrochloric acid the rate is measurable. Therefore, the reaction was carried out in a mixture of both the acids. The effect of reactants on the reaction was studied at constant [H+] and [Cl–] of 0.23 and 0.13 mol dm–3 each and ionic strength of 0.6 mol dm–3. Concentration of oxidant was varied from 6.4×10-4 to 6.4×10-3 mol dm–3 keeping the [hydrazide] constant at 1×10-1 mol dm–3.Since,the pseudo first order rate constants were fairly constant (3.6 ± 0.1×10-4 s-1 for BAH at 250C and 6.70 ± 0.1×10-4 s-1 for BAH at 250C and 2.3 ± 0.1×10-4 s-1 for p- Cl BAH at 250C, the order with respect to [oxidant] is unity. The effect of [hydrazide] was studied between the concentration range from 1×10-2 to 1×10-1 mol dm–3 keeping the [oxidant] constant at 3.0×10-3 mol dm–3.The pseudo first order rate constants increases with increase in concentration and the order with respect to hydrazide is found to be fractional (0.75 for BAH, 0.79 for o- Toluic BAH and 0.70 for p- Toluic BAH).
To study the effect of [H+] and [Cl–],[oxidant], [hydrazide] and ionic strength were kept as 3.0×10-3, 1×10-1 and 0.6 mol dm–3 respectively. To vary [H+] and [Cl–], HClO4 and NaCl were used. Increase in [H+] from 0.13 to 0.60 mol dm–3 decreases 10-4 k(s-1) from 4.20 to 0.15 for BAH at 250C and 74.20 to 1.05 for o- Toluic BAH at 250C and from 2.03 to 0.08 for p- Toluic BAH at 250C. Increase in [Cl–] from 0.13 to 0.60 mol dm–3 decreases 10-4 k(S-1) from 2.8 to 0.095 for BAH at 250C and 7.35 to 1.05 for o- Toluic BAH at 250C and from 1.40 to 0.060 for p- Toluic BAH at 250C. The relative permittivity was varied by changing the 1,4-dioxan content from 5 to 40 % v/v. The rate was found to decrease with decrease in relative permittivity.
Added acrylonitrile in the concentration range 0.5 to 2.5vol.% by keeping concentrations of oxidant,reductant,perchloric acid,hydrochloric acid and ionic strength fixed did not produce any precipitate due to polymerization of the added acrylonitrile on the pseudofirst order rate constants indicating absence of free radicals.
Since there is no formation of free radicals in the reaction, the reaction proceeds with two-electron transfer step. The order in thallium (III) was found to be unity and the order in hydrazide was found to be fractional. Such fractional order in substrate concentration is due to the prior complex formation equilibrium between the reactants.
![]()
Complex ® TlI + Intermediate kl
TlIII+Intermediate ® TlI + Products fast
Scheme 1
The Michealis – Menten plots of 1/kobs versus 1/[Hydrazide] were linear with an intercept in support of the complex formation. Therefore, in agreement with the results obtained the mechanism of the reaction can be represented as in Scheme 1. Equation 2 gives the rate according to Scheme1. Since, total [TlIII] exists in the form of free [TlIII] and the complex (Equation 3) therefore, the [TlIII] free is given by Equation 6. The overall rate law is now expressed by Equation 7 and the Pseudo-first order rate constant kobs, by Equation 8.
Rate = k1 [Complex] = k1Kc [Hydrazide] free [TIIII] free (2)
[TIIII] total = [T1III] free+ [Complex] (3)
[TIIII] total = [T1III] free + Kc [Hydrazide] [TIIII] free (5)
[T1III] free = [TIIII] total/(1+ Kc [Hydrazide]) (6)
Rate = k1Kc [Hydrazide] [TIIII] free (7)
kobs = k1Kc [Hydrazide]/(1 + Kc [Hydrazide]) (8)
Rate law 8 is verified by plotting 1/kobs against 1/[Hydrazide] at four different temperatures and from the slopes and intercepts of these plots the values of k1 and Kc were calculated and are given in Table 1.
The effect of hydrogen and chloride ion concentrations on the reaction is due to the protonation of hydrazides11and different chloro – complexes12 of thallium (III) present in the solution. in acid medium according to Equation 9. Hydrazides are known to be protonated, therefore, total [Hydrazide] can be expressed by Equation 10 and thereby the fact that there was no effect of Free [Hydrazide] by Eq. 12. Since the rates of reaction decreases as the [H+] increases, free hydrazide is the active species, this is in support of ionic strength on the reactions indicating one of the reactant is neutral.
![]()
[Hydrazide] total = [Hydrazide] free + [Hydrazide]protonated (10)
[Hydrazide]total = [Hydrazide]free + KH [Hydrazide]free (11)
[Hydrazide]free = [Hydrazide]total /(I+ KH [H+]) (12)
Thallium (III) forms strong complexes with chloride ions of the formula TlCln3-n where n is the number of chlorides complexes with thallium(III) as represented in equilibrium 13 to 16. The values of respective stability constants13 are K1 = 1.38 X 108, K2 = 3.98 X 1013, K3 = 6.02 X 1015 and K4 = 1.0 X 1018 mol–1dm3.

All the thallium(III) will exists as TlCl2+ and its concentration can be expressed by Equation 17. The [TlCl2]+free can now be given by eq. 19 where, b1 = K3/K2 = 151 and b2 = K4/K3 = 166, further, using Equations 18 and 19 the concentrations of [TlCl2]+free, TlCl3 and TlCl4– were calculated at different chloride ion concentrations and compared with the change in rate constant as the chloride ion concentration varied.
[TI (III)] total = [T1CI2+] total = [T1C12+] free + [T1C13] + [T1C14] (17)
[T1C12+] total = [T1C12+] free (1+ β[Cl–] + β2[Cl–]2) (18)
[T1C12+] free = [T1Cl2+] total / (1 + β[Cl–] + β2[Cl–]2) (19)
The concentration of both of [TlCl2+] free and TlCl3 parallel the values of rate constants as [Cl–] changes but the order [Cl–] is – 1.5, which makes [TlCl2+] free as the only active species.
![]()
Complex → RCONNH + T1C12– + H+ k1
RCONNH+H20+T1C12+ → RCOOH+N2+2H+ + T1C12– fast
where R = Alkyl group for acid hydrazides
Scheme 2
The mechanism considering TlCl2+ of oxidant and free hydrazide of the substrate as the active species can now be represented by scheme 2 with respective rate law and the expression for the pseudo-first order rate constants by Equations 20 and 21. The rate law 21 was verified by plotting 1/kobs against 1/[Hydrazide] and 1/kobs against [H+] which were found to be linear. From the slopes and intercepts of these plots the values of Kc and KH were determined. The values of Kc are given in Table I and those of KH were found to be 13 and 16 mol-1 dm3 for heterocyclic acid hydrazides respectively.
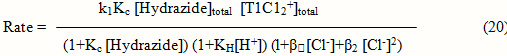
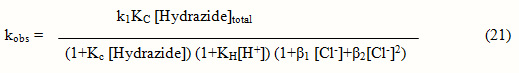
The electrophilic character of TlCl2+ among the thallium (III) chlorocomplexes is highest thus making it the reactive species.
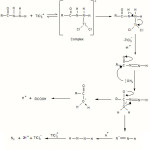 |
Scheme 3 |
The detailed mechanism involves electrophilic substitution on the nitrogen of the hydrazide with the formation of N-Tl bond, which decomposes in the subsequent step with, direct two-electron transfer from hydrazide to thallium to give an intermediate followed by fast steps. (Scheme 3). Such N-T1 bond formation has been postulated during thallium (III) oxidation of nitrogen14 containing compounds.
The activation parameters, with respect to slow step, k1, D H# (KJ mol-1), DG# (KJ mol-1) and DS# (JK-1mol-1) were found to be 59.74 , 97.69 and –94.64 respectively for benzoic acid hydrazide ( BAH) and 36.78 ,78.11 and –204.38 for p- methoxy benzoic acid hydrazide (o- ToluicBAH) and 71.82 , 98.80 and –90.54 respectively for benzoic, p- methoxy benzoic and p- chloro benzoic acid hydrazide (p- Toluic BAH). Considerable decrease in the entropy of activation is due to formation of more ordered transition state as shown in scheme 3. The mechanism involves neutral hydrazide as the active substrate thus the reaction is unaffected by the change in the ionic strength. The increase in 1,4-dioxan content in the reaction medium decreases; the rate such an effect of the solvent is due to the stabilization of the complex formed between reactants15 in a medium of low relative permittivity.
Conclusion
The order of reactivities of Benzoic and substituted benzoic acid hydrazides under investigation is –
p – Cl BAH < BAH < o- Toluic BAH
The observed sequence can be attributed to various possibilities and factors. The highest rate of o- Toluic BAH is due to strong electron donating mesomeric effect of -OCH3 group since it is in the para position, the unsubstituted benzoic acid hydrazide has intermediate stability.
Chlorine has electron withdrawing inductive effect but an electron-donating mesomeric effect since it is in the para position.
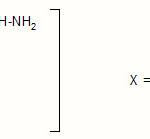 |
Scheme 4 |
Hence, it is evident that the rate of reaction is enhanced by electron donating group(s) at para position and retarded by electron withdrawing group(s).
Acknowledgement
The authors are thankful to Principal Dr. S.T.Gadade for his keen interest and help during the course of work.
Table 1: Kc (mol-1dm3) and k1 (mol-1dm3) T = 288 K to 303 K
|
Temp |
KC |
k x 104 s-1 |
KC (mol-1dm3) |
k x 104 s-1 |
KC |
k x 104 s-1 |
0C |
(mol-1 dm3) |
BAH |
o- ToluicBAH |
o- Toluic |
(mol-1dm3) |
p- Toluic |
|
|
BAH |
BAH |
p- Toluic BAH |
BAH |
||
|
15 |
59.09 |
1.23 |
7.42 |
0.10 |
16.66 |
1.25 |
|
20 |
55.50 |
2.05 |
8.00 |
0.12 |
17.50 |
2.38 |
|
25 |
47.69 |
2.58 |
7.00 | 0.16 |
15.00 |
3.33 |
|
30 |
40.00 |
4.44 |
7.57 |
0.22 |
17.50 |
10.00 |
References
- a) Ho. T.L. and Ho. H. C., Wong, C. M.; Synthesis., 21, 562-568 (1972). b) Schnyder J. and Rttenberg, M. Helv. Chim. Acta,, 58, 521-527(1975). c)Tsuji J. Takayanagi H. Toshida Y. Chem. Lett., 12, 147-153 (1976).
- a) Aylward J. B. Norman R. O. C. J. Chem. Soc.(C)., 26, , 2399-2408 (1968). b)Tsuji J. Hayakawa H. Takayanagi, Chem. Lett, 18, 437-445 (1975). c) Hoffman R. V. Kumar A. J. J. Org. Chem. 49, 4014-4020 (1984).
- Werner W. J org chem, 18, 1333-1338 (1953).
- Madzhoyan A.L. Arm Khim Zh,, 19, 793-799 (1966),.
- Winterstein, A. Hegedus Fust, B. Bohni E. Studer A.Helv.Chem.Acta., 39, 229-235 (1956).
- Pfister R. Soilman A. Chimat Hammers W. Chem Abstract., 68, 283-294(1968).
- Jucker F. Linde, A. Helv chim Acta., 45, 2316-2325 (1962).
- Radhakrishnamurthi P.S. Patil S.N. J.chem., 17A, 97-104 (1979).
- Radhakrishnamurthi P.S. Patil S.N. J.chem., 16A, 139-142 (1978).
- Vogel A. I. Textbook of practical organic chemistry (ELBS &Longman Group) 4, 1125-1135 (1975).
- Weast R. C. Handbook of chemistry and physics,edited by,50th edn.(CRC) 12, 112-116 (1970).
- (a) Kazo K. Hirakazo T. Hisashi K. Zenzo, T. Chem. Pharm. Bull., 11, 797-808 (1963). (b) Krishnarao P. V. Frank, M. S. Ramaih A. K. React. Kinet. Cata.. Lett, 9, 59-65 (1978).(c) Krishnarao P.V. Frank, M. S. Ramaih A.K. Indian J. Chem, 16A ,418-425 (1978)..(d) Ramaih A.K. Frank M. S Baburao G. Krishnarao, P.V. Indian J. Chem., 18A, 416-429. (1979).
- Lee A. G. The Chemistry of Thallium, ( Elsevier, London) 17, 48-52 (1971)
- (a) Mckillop A. Hunt, J.D. Naylor R. D. Taylor, E. C. J. Am. Chem., 93, 4918- 4925 (1971). (b) Buttler R.N. Morris G. J. Donohue A. M. O J. Chem. Res. (s).Soc., 12, 61-67 (1981).
- Amis E. S. Solvent effects on reaction rates and mechanisms, (AcademicPres, New York) 12, 145-48 (1966).
- Varale A.S. Hilage N.P. Oxidation Communication.,31,537-545 (2009).
- Varale A.S. Hilage, N.P. Asian Journal of Chemistry., 21, 1965-72 (2009)
- Varale A.S. Hilage, N.P. Oriental Journal of Chemistry., 24, 545-550 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









