A Regioselective and Stereoselective Methoxy Bromination of Olefins using Diacetoxyiodobenzene and Phenyltrimethyl Ammoniumtribromide
S. P. Hangirgekar1* and S.G. Shirodkar2
1School of Chemical Sciences, Swami Ramanad Teerth Marathwada University, Nanded - 431 606 (India).
2Department of Chemistry, Netaji Subhashchandra Bose College, Nanded (India).
A facile regio and stereoselective methoxy-bromination of alkenes using phenyltrimethyl ammoniumtribromide (PTAB) and (diacetoxyiodo) benzene (DIB) as oxidant has been carried out in the present investigation. The IR, NMR and LCMS of all the synthesized compounds have been used to characterize them.
KEYWORDS:Methoxy-bromination; Hypervalent iodine; Phenyltrimethyl ammonium tribromide; Alkene and Oxidation
Download this article as:| Copy the following to cite this article: Hangirgekar S. P, Shirodkar S. G. A Regioselective and Stereoselective Methoxy Bromination of Olefins using Diacetoxyiodobenzene and Phenyltrimethyl Ammoniumtribromide. Orient J Chem 2011;27(1). |
| Copy the following to cite this URL: Hangirgekar S. P, Shirodkar S. G. A Regioselective and Stereoselective Methoxy Bromination of Olefins using Diacetoxyiodobenzene and Phenyltrimethyl Ammoniumtribromide. Orient J Chem 2011;27(1). Available from: http://www.orientjchem.org/?p=24802 |
Introduction
The transformation of olefins into the corresponding alkoxy-bromides is frequently practiced in organic synthesis1. The resulting bromo-derivatives of olefins are precursors to organometallic reagents and useful in nucleophilic substitution reactions2. These bromo-derivatives also serve as key intermediates in the synthesis of several marine natural products, drugs, agrochemicals, pigments and photographic materials3.
The addition of molecular bromine to an alkene in methanol solvent is a traditional method for the generation of vicinal methoxy-bromides4. To overcome the drawbacks of handling and toxicity issues of molecular bromine, several other solid brominating agents such as N-bromosuccinimide5, N-bromoacetamide6, and N, N-dibromo-p-toluenesulphonamide (TsNBr2)7 have been used under different reaction conditions. Alternatively, the generation of electrophilic bromonium ion from bromide salts or HBr, under oxidative conditions, has also resulted in bromo functionalization of olefins. The various oxidizing agents reported for the generations of bromonium ion from bromide salts are CAN8, oxone9, HNO310, NaIO411 and H2O212.
Although all of these methods that are known for the formation of alkoxybromides, some of them are associated with the formation of side products, use of expensive and toxic oxidants, poor stereoselectivity and low yields of the products. Thus, there is still a need for the development of a new and efficient method for the synthesis of vicinal alkoxy-bromides.
The last two decades have witnessed an exponential growth in the applications of hypervalent iodine reagents in organic synthesis13. (Diacetoxyiodo)benzene (DIB) is the most extensively utilized parent hypervalent iodine (III) reagent14. It is easy to handle, nontoxic, commercially available and is similar in reactivity to heavy metal reagents and anodic oxidation15. The combination of DIB with trimethylsilyl bromide or quaternary ammonium bromide salts has been previously reported for electrophilic bromination of selected substrates16-20. Rho has reported the use of DIB and TMSBr16a or Bu4NBr16b for the 3-bromintion of flavones, and Evans used the former in the related bromination of dihydropyrans17. The bromoacetoxylation of glycals using polymer bound R4NBr18, of cyclic olefins with tetraethylammonium bromide19, and of 1,4-dimethoxynaphthalenes using TMSBr have also been reported20. Recently, Zhdankin et al. have reported the use of recyclable hypervalent iodine such as 4,4’-bis(dichloroiodo)biphenyl and 3-(dichloroiodo)benzoic acid for the vicinal halomethoxylation of unsaturated compounds 21.
In present paper we would like to report a mild method for the regio and stereoselective methoxy-bromination of olefins using DIB and phenyltrimethyl ammonium tribromides (PTAB) (Scheme 1):
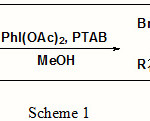 |
Scheme 1 |
Experimental Section
Melting points are uncorrected. IR spectra were recorded on a Perkin-Elmer FTIR-1710 spectrophotometer. 1HNMR spectra were recorded at 400 MHz in CDCl3 using TMS as internal standard.
General Procedure for Methoxy-Bromination of Olefins
To a stirred mixture of olefin (1 mmol) and PTAB (1mmol) in MeOH (10 mL) at room temperature, (diacetoxyiodo)benzene (1 mmol) was added. The reaction mixture immediately turned yellow. The progress of the reaction was monitored by TLC. After completion of the reaction, it was diluted with water and extracted with CH2Cl2 (15 mL x 2). The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give crude products, which were purified by column chromatography packed with silica gel using petroleum ether and ethyl acetate (9:1) as eluent to afford the pure products.
Results and Discussion
The reaction of cyclohexene with DIB/PTAB in MeOH was chosen a model reaction. When 1:1 stoichiometric ratio of PTAB and DIB was added to cyclohexene in MeOH, the reaction mixture immediately turned yellow, which slowly disappeared as the reaction progressed towards completion. The usual aqueous work-up of reaction mixture resulted in the formation of 1-bromo-2-methoxy-cyclohexane in 80% yield. The 1H NMR analysis of this product has revealed that the protons attached to carbon bearing bromine and methoxy-groups appear as quartet at d 3.78 and d 2.90 respectively. Having succeeded with this result, wide range of olefins was subjected to methoxy-bromination reaction and the results are summarized in Table 1.
The present procedure for methoxy-bromination reaction of olefins is quite general as a wide range of olefins such as terminal, internal, cyclic and acyclic olefins underwent oxidative addition under exceptionally mild conditions. The addition reaction is completely regioselective and stereoselective. The orientation of the reaction for the terminal olefins such as styrene, α-methyl styrene and 1-hexadecene obeyed Markovnikov rule to form respective products in which bromine is bonded to the terminal carbon. The stereochemical mode of addition was found to be anti as revealed by the coupling constant analysis of 1H NMR spectra of methoxy-bromination products. In all cases the erythro isomers were formed.
A probable mechanistic pathway to explain the regio and stereoselectivity of the methoxy-bromination reaction is depicted in Scheme 2. The ligand exchange reaction between DIB and PTAB will form a putative intermediate 3. The inherent tendency of intermediate 3 for the reductive elimination of iodobenzene will make it an active source of electrophilic bromine. Thus, the intermediate 3 with the concomitant reductive elimination of iodobenzene15 can react with olefins to form a cyclic three membered bromonium ion intermediate 4. The intermediate 4 undergoes ring opening by nucleophilic methanol solvent via SN2 pathway to form vicinal methoxy-bromides. The SN2 opening is responsible for the high anti stereoselectivity of the various bromo products. This assumption can again be realized from the coupling constants of protons attached to the carbon bearing bromine and methoxy group in the NMR spectra of various products. The regioselectivity can be explained by considering the relative stability of incipient carbocation generated during ring opening reaction of bromonium ion 4.
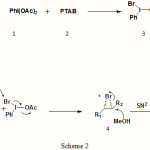 |
Scheme 2 |
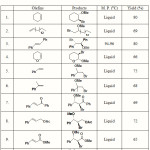 |
Table 1: Synthesis of Vicinal-Methoxy Bromides from Olefins Using DIB/PTAB in Methanol |
Spectral data for selected products:
1-Bromo-2-methoxyhexadecane (2b): IR (KBr): 2939, 2872, 2837, 1461, 1389, 1192, 1103 cm-1. 1HNMR (CDCl3): d = 0.89 (t, J = 3.3 Hz, 3H), 1.28 (m, 26H), 3.37 (s, 3H), 3.43 (m, 2H), 3.63(quintet, J = 6.3 Hz, 1H). LCMS (m/z): 336 (M+1).
1-Bromo-2-methoxy-1,2-diphenyl ethane (3b): IR (KBr): 3012, 2929, 2891, 1656, 1598, 1494, 1453, 1319, 1278, 1217, 1095, 764, 698 cm-1. 1HNMR (CDCl3): d = 3.19 ( s, 3H ), 4.64 ( d, J = 6.92 Hz, 1H ), 5.03 ( d, J = 6.92 Hz, 1H ), 7.22-7.35 ( m, 10H ). LCMS (m/z): 291 (M+1).
3-Bromo-tetrahydro-2-methoxy-2H-pyran (4b): IR (KBr): 3018, 2960, 2927, 2854, 1458, 1261, 1215, 1095, 1016, 793 cm-1. 1HNMR (CDCl3): d = 1.55 (m, 2H), 1.94 (m,2H), 4.50 (d, J = 4.64, 1H), 3.61 (m, 1H), 3.45 (s, 3H), 3.91 (m, 2H). LCMS (m/z): 196 (M+1).
1-(2-Bromo-1-methoxyethyl)benzene (5b): IR (KBr): 3020, 2943, 2881, 2820, 1590, 1500, 1470, 1351, 1212, 1110, 1090, 776 cm-1. 1HNMR (CDCl3): d = 3.29 ( s, 3H ),3.47( dd, J = 4.32, 1.88 Hz, 1H ), 3.52( dd, J = 8.24, 8.24 Hz, 1H ), 4.38( dd, J = 4.28, 4.24 Hz, 1H ), 7.37-7.40 ( m, 5H ). LCMS (m/z): 216 (M+1).
1-(1-Bromo-2-methoxypropan-2-yl)benzene (6b): IR (KBr): 3020, 2882, 2827, 1601, 1507, 1457, 1391, 1278, 1207, 1081, 781, 752 cm-1. 1HNMR (CDCl3): d = 1.71 ( s,3H ), 3.14 ( s, 3H ), 3.51( d, J = 10.64Hz, 1H ), 3.62 ( d, J =10.84 Hz, 1H ), 7.29-7.37 ( m, 5H ). LCMS (m/z): 230
(M+1).
2-Bromo-3-methoxy-1,3-diphenylpropan-1-one (7b): IR (KBr): 3062, 2928, 2821, 1691, 1642, 1594, 1458, 1259,1097, 810, 710 cm-1. 1HNMR (CDCl3): d = 3.19 (s, 3H), 4.85(d, J = 9.84, 1H), 5.13 (d, J = 9.84 Hz, 1H), 7.63 (m,5H), 8.01 (m, 5H). LCMS (m/z): 320 (M+1).
2-Bromo-3-methoxy-3-phenylpropyl acetate (8b): IR (KBr): 3050, 2982, 2853, 1758, 1620, 1570, 1601, 1507, 1570, 1465, 1230, 763 cm-1. 1HNMR (CDCl3): d = 2.01 ( s, 3H ), 3.21 ( s, 3H ), 4.41-4.45 (m, 4H), 7.38 ( m, 5H ). LCMS (m/z): 288 (M+1).
3-Bromo-4-methoxy-4-phenylbutan-2-one (9b): IR (KBr): 3027, 2881, 1771, 1760, 1620, 1425, 1361, 1238, 1192, 792 cm-1. 1HNMR (CDCl3): d = 3.21 (s, 3H), 3.84 ( s,3H), 4.23(d, J = 9.92, 1H), 4.55(d, J = 9.92 Hz, 1H), 7.38 (m, 5H). LCMS (m/z): 274 (M+1).
Conclusion
In conclusion, we have demonstrated a mild oxidative methoxy-bromination reaction of olefins using environmentally benign (diacetoxyiodo) benzene as oxidant and solid brominating agent PTAB. Apart from being completely regio and stereoselective course of the reaction, this methodology is characterized by high yields of the products, short reaction time and easy work up procedure.
References
- (a) House, H. Modern Synthetic Reactions, 2nd ed.; W. A. Benjamin: Menlo Park, CA, 432, (1972). (b) March, J. Advanced Organic Chemistry, 4th ed.; Wiley: New York, 815 (1992). (c) Eisch, J. J.; Liu, Z. R.; Ma, X.; Zheng, G. X. J. Org. Chem. 1992, 57, 5140.
- (a) Davies, S. G. In Organotransition Metal Chemistry: Applications to Organic Synthesis: Pergamon: Oxford, (1982). (b) Sasson, Y. Formation of Carbon-Halogen Bonds (Cl, Br, I). In The Chemistry of Functional Groups, Supplement D2: The Chemistry of Halides, Pseudo Halides and Azides, Part 2; Patai, S.; Rappoport, Z., Eds.; Wiley: Chichester, 535 (1995). (c) Yang, X.; Althammer, A.; Knochel, P. Org. Lett., 6, 1665 (2004).
- (a) Fenical, W. In Marine Natural Products; Scheuer, P. J., Ed.; Academic: New York, 2, 174, (1980). (b) Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Electronic release, Wiley VCH: Weinheim, Germany, (1998). (c) Cabanal-Duvillard, I.; Berrier, J. F.; Royer, J.; Husson, H. P. Tetrahedron Lett., 39, 5181 (1998).
- Curtin, D. Y.; Meislich, E. K. J. Am. Chem. Soc., 74, 5518 (1952).
- Dulcere, J. P.; Rodriguez, J.; Santelli, M.; Zahara, J. P. Tetrahedron Lett. 1987, 28, (2009).
- Buckles, R. E.; Filler, R.; Hilfman, L. J. Chem. Soc. Perkin Trans. 1., 1877 (1992).
- Phukan, P.; Chakraborty, P.; Kataki, D. J. Org. Chem, 71, 7533 (2006).
- Roy, S. C.; Guin, C.; Rana, K. K.; Maiti, G. Synlett., 2, 226 (2001).
- (a) Dieter, R. K.; Nice, L. E.; Velu, S. E. Tetrahedron Lett., 37, 2377 (1996). (b) Martinez-Perez, J. A.; Pickel, M. A.; Caroff, E.; Woggon, W. D. Synlett., 1875 (1999).
- Joshi, A. V.; Baidossi, M.; Mukhopadhyay, S.; Sasson, Y. Org. Process Res. Dev., 8, 568 (2004).
- Dewkar, G. K.; Narina, S. V.; Sudalai, A. Org. Lett., 23, 4501(2003).
- (a) Mukhopadhyay, S.; Ananthakrishnan, S.; Chandalia, S. B. Org. Process. Res. Dev., 3, 451(1999). (b) Dakka, J.; Sasson, Y. J. Chem. Soc. Chem. Commun., 1421 (1987).
- (a) Wirth, T. Angew Chem. Int. Ed., 44, 3656 (2005). (b) Moriarty, R. M. J. Org. Chem., 70, 2893 (2005). (c) Stang, P. J. J. Org. Chem., 68, 2997 (2003). (d) Zhdankin, V. V.; Stang, P. J. Chem. Rev., 102, 2523 (2002). (e) Moriarty, R. M.; Prakash, O. Org. React., 57, 327 (2002).
- Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: London, Chapter 3, 19 (1997).
- (a) Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Eur. J. Org. Chem, 1064 (2003). (b) Boye, A. C.; Meyer, D.; Ingison, C. K.; French, A. N.; Wirth, T. Org. Lett., 5, 2157 (2003). (c) Kawamura, Y.; Maruyama, M.; Tokuoka, T.; Tsukayama, M. Synthesis, 2490 (2002).
- (a) Rho, H. S.; Ko, B. S.; Ju, Y. S. Synth. Commun., 31, 2101 (2001). (b) Rho, H. S.; Ko, B. S.; Kim, H. K.; Ju. Y. S. Synth. Commun., 32, 1303 (2002).
- Evans, P. A.; Nelson, J. D.; Manangan, T. Synlett., 968 (1997).
- (a) Evans, P. A.; Brandt, T. A. Tetrahedron Lett., 37, 6443 (1996). (b) Evans, P. A.; Brandt, T. A. J. Org. Chem., 62, 5321 (1997).
- Kirsching, A.; Jesberger, M.; Monenschein, H. Tetrahedron Lett., 40, 8999 (1999).
- Hashem, M. A.; Jung, A.; Ries, M.; Kirsching, A. Synlett., 195 (1998).
- Yusubov, M. S.; Drygunova, L. A.; Zhdankin, V. V. Synthesis, 14, 2289 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









