Voltammetric Behaviour of Metronidazole at A Composite Polymer Membrane Electrode
Gaurav Sahu
Department of Chemistry, Jiwaji University, Gwalior - 474 001 (India).
Voltametric behaviour of metronidazole was studied in Britton Robinson buffer system at composite polymer membrane working electrode. Cyclic voltammetric method has been developed for the determination of drug in pharmaceutical formulation. A well-defined cathodic peak was observed for the metronidazole in the entire pH range. The current increases steadily with diffusion scan rate and concentration. The results indicate that the process is irreversible and diffusion controlled. This composite film showed good current response.
KEYWORDS:Metronidazole; composite polymer electrode; cyclic voltammetry
Download this article as:| Copy the following to cite this article: Sahu G. Voltammetric Behaviour of Metronidazole at A Composite Polymer Membrane Electrode. Orient J Chem 2010;26(1).. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Sahu G. Voltammetric Behaviour of Metronidazole at A Composite Polymer Membrane Electrode. Orient J Chem 2010;26(1).. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23448 |
Introduction
Nitroimidazole derivatives contain toxic selectivities to anaerobic micro-organisms1. The most important derivatives contain the 5-nitroimidazole nucleus with substituints at the N1 position of the heterocyclic aromatic ring2. Metronidazole [1-(2-hydroxyethyl)2-methyl-5-nitroimidazole] has been used as a therapeutic drug for at least 30 years. The reduction of the nitroimidazoles is a complex process, involving 6 electrons for complete reduction of the nitro group
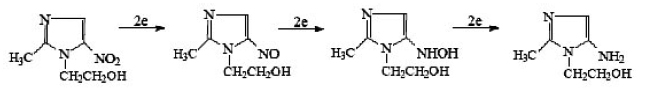
In voltammetric studies of nitroimidazole derivatives7-9, two reduction waves were observed in aqueous acid medium. The first one is due to the nitro group reduction to hydroxylamine and the last one to the subsequent reduction to the amine derivative. Generally the E1/2 values for the nitro group reduction change with the lateral chain at the to the amine derivative3 (Scheme-A). Under anaerobic conditions or low oxygen pressure, the reduction process is similar to that observed for nitrobenzene4. The reduction mechanism for several aromatic and heterocyclic nitrocompounds was presented by Zuman and co-workers5.
A total of two electrons and two protons is involved in the formation of the nitroso (R-NO) intermediate, two more electrons and protons result in the hydroxylamine(R-NHOH)6:
N1 position of the heterocyclic ring10. Some compounds showed only one reduction wave involving six electrons in alkaline medium. The polarographic behaviour of metronidazole was initially reported to be similar in neutral (pH 7.3) and acid medium, with E1/2 = -0.34 and -0.92 V vs. SCE. Later, Leach and co-workers11 observed only one cathodic wave for metronidazole with E1/2 = – 0.465 V in the same buffer solution of pH 7.3. For ornidazole two waves were also reported in acid and neutral medium with disappearance of the second cathodic wave on increasing pH. Zuman and co-workers12 showed that the E1/2 for the first reduction step of nitrocompounds is shifted to more negative potentials with increasing pH. This change in the E1/2 values occurs both in aqueous medium and solutions with different percentages of organic solvents. These shifts were attributed to an acid-base pre-equilibrium followed by the first electron transfer to form the nitro radical. Voltammetric methods have been applied to the study of the mechanism of nitroimidazoles as antimicrobial agents13, and its determination in pharmaceutical14- and clinical16-17 matrices. The mercury electrode was the most used, but good results were obtainedusing solid electrodes when studying nitrobenzene18 and metronidazole19. Barety and co-workers20 observed the stabilization of the nitro radical(R-NO2–) by using cyclic voltammetry in non aqueous medium (dimethylsulfoxide). This species may be responsible for the observed DNA damage21. The process was characterized as an ECi mechanism and the reversibility of the first step was dependent on the substituent groups on the heterocyclic ring of the nitroimidazole.
In recent years, increased interest in conducting polymers has led to a large number of important applications such as rechargeable batteries, electrosynthesis, in corrosion to protect films, biosensors, modified electrodes and capacitors22-31 etc. Conducting polymers containing two components can be prepared as copolymers32, bilayers33 and composites. Some properties of two component conductive polymer systems, such as electrical and physical properties can be improved by copolymerization. The mechanical properties of two component conductive polymeric systems may also be improved by polymerization to two monomers, on platinum foil electrodes34-37. Electrolysis of two monomers generally gives a copolymer, as in the case of aniline and thiophene. Pekmez and co-workers38 reported that the thiophene / aniline ratio in the copolymer could easily be adjusted by controlling the polymerization potential.
In the present study, the electrochemical behaviour of metronidazole has been studied at composite polymer membrane electrode. The main aim of the combination of polycomponent composites, copolymers and blends is to improve the electrical, physical and chemical properties of the homopolymers. In the present paper composite polymer membrane of polyaniline and pyrrole is used as an electrode material for the investigation of electrochemical behaviour of metronidazole. This composite polymer membrane electrode was prepared electrochemically.
Experimental
The voltammetry experiments were performed using an EG & G Princeton Applied Research Model 273A potentiostat controlled by the model 270 / 250 Research Electrochemistry Software 4.30. A three-electrode system was composed of a composite polymer membrane electrode, saturated calomel as reference electrode and platinum wire as auxiliary electrode.
The platinum foil was washed with dilute HNO3 and acetone and then coated with polymer to provide a reproducible active surface with improved sensitivity and resolution. Then composite polymer membrane electrode was thoroughly rinsed with methanol and double distilled water and gently dried with a tissue paper. The electrode cleaning procedures require only 2 min. All the solutions examined by electrochemical technique were purged for 10 min with purified nitrogen gas after which a continuous stream of nitrogen was passed over the solutions during the measurements. All measurements were carried out at room temperature. The pH metric measurements were made on digital pH meter (Decible, Db- 1011) fitted with a glass electrode and saturated calomel electrode as reference, which was previously standardized with buffers of known pH in acidic and alkaline medium.
Reagents and Materials
Metronidazole was obtained from pharmaceutical company, India. KCI (1 mol L-1) solution was prepared in distilled water and used as supporting electrolyte. A stock solution of metronidazole (l x 10-3 mol L-1) was prepared in dimethyl formamide (DMF). The solutions for recording voltammograms were prepared by mixing appropriate volume of stock solution and buffer of varying pH. Britton Robinson buffers in the pH range 2.5 – 12.0 were prepared in distilled water by adding suitable amounts of 0.4 M NaOH solution (basic solution) to a stock solution composed of a mixture of 2.14 mL phosphoric acid, 2.472 g boric acid and 2.3 mL of glacial acetic acid (acidic solution). Solution was stirred after mixing and left overnight to attain equilibrium. All reagents used were AnalaR grade.
Procedure
The amount of metronidazole present in each tablet was 100 mg. Excipients such as microcrystalline cellulose, hydroxypropyl methylcellulose, lactose and titanium dioxide are added to dosage forms. Ten tablets were weighted accurately and crushed to a fine powder. The accurately weighted quantities of these powders equivalent to 2.5 g of metronidazole were transferred to 50 mL volumetric flask and dissolved in dimethylformamide. After sonicating and shaking the mixture for 30 min, it was completed to volume with the same solvent, mixed and centrifuged. An aliquot of the supernatant liquid was then transferred into a calibrated flask and a series of dilutions were prepared with BR buffers at pH 2-12 and mixed 1 mL KCI as supporting electrolyte.
Results and Discussion
The voltammetric behaviour of metronidazole was examined in the pH range 2.0-12.0 by cyclic voltammetry. Metronidazole gave one well-defined cathodic peak in the entire buffer system at composite polymer membrane electrode. Cyclic voltammograms of metronidazole were also recorded in dimethylformamide at composite polymer electrode at varying scan rates (20–200 mV/s). Cyclic voltammograms show a single reduction peak at -784 mV at 20 mV/s. As the scan rate increased, the cathodic peak potential shifted towards negative potential -930 mV at 200 mV/s (Figure 1). Plot of ip,c vs. ul/2 (u = scan rate) is a straight line passing through the origin indicating diffusion controlled nature of the electrode process (Figure 2). With the increase in concentration of the drug a linear relation between peak current and concentration was observed in the range from 1×10-3 to 4×10-3 at composite polymer membrane electrode. The peak potential (Ep,c) shifted toward more negative potential (from -784 to -859.9 mV) on increasing the concentration of the metronidazole, indicating the irreversibility of the electrode process. Plot of ip,c vs. concentration is a straight line at composite polymer membrane electrode (Figure 3).
Cyclic voltammograms using composite polymer membrane electrode were also recorded at different pH. The pH of the solution was varied from 2.0 to 12.0. No anodic peak in the entire pH range was observed. The peak potential values of metronidazole are dependent on pH and peak current increases with increase in the pH of the buffer system. The influence of several electrolytes (Phosphate & Britton- Robinson) on the analytical signal was also studied. With the rise in pH peak potential shifted towards more negative values (Figure
up to pH 7.9 indicating the participation of proton in the electrode process.
Reaction Mechanism
On the basis CV, chromatographic and spectral studies following mechanism may be postulated for the reduction of metronidazole. The addition of two more electrons results in formation of the amine.
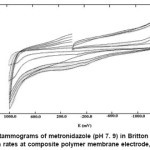 |
Figure 1: Cyclic voltammograms of metronidazole (pH 7. 9) in Britton Robinson buffer at different scan rates at composite polymer membrane electrode, Conc. 1×10-3 M Click here to View figure |
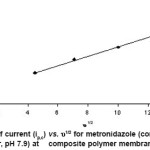 |
Figure 2: Plot of current (ip,c) vs. υ1/2 for metronidazole (conc. 1.0 x 10-3 M, BR buffer, pH 7.9) at composite polymer membrane electrode Click here to View figure |
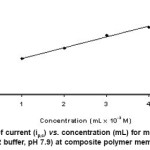 |
Figure 3: Plot of current (ip,c) vs. concentration (mL) for metronidazole (υ = 50 mV/s, BR buffer, pH 7.9) at composite polymer membrane electrode Click here to View figure |
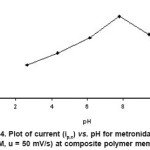 |
Figure 4: Plot of current (ip,c) vs. pH for metronidazole (conc. 1.0 x 10-3 M, u = 50 mV/s) at composite polymer membrane electrode. Click here to View figure |
Conclusion
Electrochemically deposited composite polymer membrane electrode on the platinum foil indicates good catalytic current response for the voltammetric determination of metronidazole drug. The current-voltage characteristic of the composite film was found to be linear which indicates a semiconducting behaviour. This composite polymer electrode also shows good stability with the solvent.
References
- Edwards, D.I. J. Antimicrob. Chemothr. 31: 9 (1993).
- Edwards, D.I. In Comprehensive Medicinal Chemistry, Hansch, C., Ed.; Pergamon Press, New York, Vol. 2, 5th ed., 725 (1990).
- Knox, R.I.; Knight, R.C.; Edwards, D.I. Biochem. Pharmac. 32: 2149 (1983).
- Zuman, P.; Fijalek, Z. J. Electroanal. Chem. 296: 538 (1990).
- Zuman, P.; Fijalek, Z.; Dumanovic, D.; Suznjevic, D. Electroanalysis 4: 783 (1992).
- Dumanovic, D.; Suznjevic, D.; Erceg, M.; Zuman, P. Electroanalysis 4: 795 (1992).
- Dumanovic, D.; Volke, J.; Vajgand, V. J. Pharm. Pharmac. 18: 507 (1966).
- Dumanovic, D.; Perkucin, S.; Volke, J. Talanta, 18: 675 (1971).
- Dumanovic, D.; Ciric, J. Talanta, 20: 525 (1973).
- Fonseca, J.M.L.; Rivera, M.C.G.; Monteagudo, J.C.G. Anal. Lett., 26: 109 (1993).
- Leach, S.C.; Weaver, R.D.; Kinoshita, K.; Lee, W. J. Electroanal. Chem. 129: 213 (1981). Chien, Y.W.; Mizuba, S.S. J. Med. Chem. 21: 374 (1978).
- Zuman, P.; Dumanovic, D.; Jovanovic, J.; Suznjevic, D.; Erceg, M. Electroanalysis, 4: 871 (1992).
- Papas, A.N.; Delaney, M.F. Anal. Lett., 15: 739 (1982).
- Zuhri, A.Z.A.; Al-Khalil, S.I.; Suleiman, M.S. Anal. Lett. 19: 453 (1986).
- Patriarche, G.J.; Vire, J.C. Anal. Chim. Acta, 196: 193 (1987).
- Brooks, M.A.; D’Arconte, L.; Silva, J.A.F. J. Pharm. Sci., 65: 112 (1976).
- Wang, Z.; Zhou, H.; Zhou, S. Talanta, 40: 1073 (1993).
- Rubinstein, I. J. Electroanal. Chem., 183: 379 (1985).
- Bishop, E.; Hussein, W. Analyst, 109: 759(1984).
- Barety, D.; Resibois, B.; Vergoten, G.; Moschetto, Y. J. Electroanal. Chem., 162: 335 (1984).
- Moreno, S.N.; Mason, R.P.; Muniz, R.P.A.; Cruz, F.S.; Docampo, R. J. Biol. Chem., 258: 4051 (1983).
- Lui, G.; Seolee, J.; Kim, S.J.; Nam, H.; Cha, G.S.; Kim, H.D. Analyst, 123: 1855 (1998).
- Shrivastava, A.; Singh, V.; Dhand, C. Analyst, 6: 262 (2006).
- Vishnuvardhan, T.K.; KulKarni, V.R.; Basavaraja, C.; Raghavendra S.C. Bull. Mater. Sci., 29: 77 (2006).
- Koleli, F.; Dubukcu, M.; Arslan, Y. Tark. J. Chem., 24: 333 (2000).
- Kassim, A.; Basar, Z.B.; Mahmud, H.N.M.E. Indian Acad. Sci. (Chem. Sci.) 114: 155 (2002).
- Ibrahim, M.; Koglin, E. Acta. Chim. Slov., 52: 159 (2005).
- Gaikward, P.D.; Shirale, D.J.; Gade, V.K.; Savale, P.A.; Kakde, K.P.; Kharat, H.J.; Shirsat, M.D. Bull. Mater, 29: 417 (2006).
- Ion, R.M.; Scarlat, F.; Scarlat, F.; Nicalescu, V.I.R. J. Optoelectronic Ad. Mater, 5: 109 (2003).
- Singh, R.; Tandon, R.P.; Chandra, S. J. Appl. Phys., 70: 243 (1991).
- Valsangiacom, C.; Sima, M.; Predoi, O.; Plapciam, C.; Kuncser, C.; Schinteie, G.; Bulinski, M. Romanian Reports in Physics, 56: 645 (2004).
- Karakisla, M.; Sacak, M. J. Polym. Sci. A Polym. Chem., 38, 51 (2000).
- Wysock, M.; Winkler, K.; Balch, A.L. J. Mater Chem., 14: 1036 (2004).
- Vermeir, I.E.; Kim, N.Y.; Laibinis, P.E. Appl. Phys. Letters, 74: 3860 (1999).
- Mahmud, H.N.M.E.; Kassim, A.; Zainal, Z.; Yunus, W.M.M. Science Asia, 31: 313 (2005).
- Planes, J.; Wolter, A.; Cheguettine, Y.; Pron, A.; Genond, E.; Nechtschein, M.; Physical Review B, 58: 7774 (1998).
- Murugendrappa, M.V.; Khasim, S.; Pradad, M.V.N.A. Bull. Mater Sci., 28: 565 (2005).
- Pekmez, K.; Pekmez, N.; Yilzid, A. Tr. J. Chem., 22: 47 (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









