Thermodynamics and Stability of Co2+, Ni2+, Cu2+ And Zn2+ Complexes With DL-2-Amino Butanedioic Acid
Sharda Soni1* and K. D. Gupta2
1Department of Chemistry, Swami Keshvanand Institute of Technology, M and G, Jaipur - 302 025 (India).
2Department of Chemistry, Malaviya National Institute of Technology, Jaipur - 302 017 (India).
pH metric titration technique has been employed to study the formation and composition of complexes of divalent Co, Ni, Cu and Zn with DL-2-amino butanedioic acid. Formation curve reveals the formation of 1:1 and 1:2 metal ligand complexes in the pH range 4.30-8.85. Their stability constant have been determined at 20°C, 30°C and 40°C and a different ionic strength viz. 0.05M, 0.1M, 0.15M and 0.2M NaClO4 by Irving Rossotti method which follow the order Co2+ < Ni2+ < Cu2+ > Zn2+ which is in agreement with Irving Williams series. The change in thermodynamic parameter DG, DH and DS accompanying complexation have been evaluated at 30°C. The stability constant and thermodynamic function of these metal complexes are discussed in relation to ionic radii, electronegativity and ionization potential of title metals.
KEYWORDS:Thermodynamics; stability of Co2+; Ni2+; Cu2+ and DL-2-amino butanedioic acid
Download this article as:| Copy the following to cite this article: Soni S, Gupta K. D. Thermodynamics and Stability of Co2+, Ni2+, Cu2+ And Zn2+ Complexes With DL-2-Amino Butanedioic Acid. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Soni S, Gupta K. D. Thermodynamics and Stability of Co2+, Ni2+, Cu2+ And Zn2+ Complexes With DL-2-Amino Butanedioic Acid. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=234538 |
Introduction
From the survey of chemical literature1-9 it has been found that , the scanty work has been reported on the behaviour and metal complexes of DL-2-amino butanedioic acid as such the present work has been under taken reporting the formation, composition and stability constant of Co2+, Ni2+, Cu2+ and Zn2+ complexes at different temperature 20°C, 30°C and 40°C and varying ionic strengths 0.05 M, 0.1 M, 0.15 M and 0.2 M by pH metric titration technique. The thermodynamic function viz. free energy (∆G), enthalpy (∆H), and entropy (∆S) of the complexation reaction have also been determined and explained.
The stability constants and thermodynamic parameters of these metal complexes are discussed on the basis of several physical properties of the title metals such as ionic radii, electronegativity and second ionization potential of the title metal ion.
Experimental
DL-2-amino butanedioic acid was obtained from E. Merck Nickel sulphate, Copper sulphate, Cobalt chloride, Zinc chloride and other reagents of Anala–R B.D.H. grade.
Procedure
pH measurements were made on a EC digital pH-meter (accuracy ± 0.01 pH) with combined glass-calomel electrode assembly in a nitrogen atmosphere. The instrument was standardized with standard buffers. A thermostat having an accuracy of ± 0.1°C maintained the temperature of the cell. Requisite amounts of NaClO4 were added to the solution mixture for maintaining the ionic strength.
The experimental procedure as described earlier (1) involves a series of pH titration of ligand with 0.1M NaOH in the absence and presence of metal ions at different metal ligand ratio, keeping the total volume 25 ml in each case. For qualitative detection of complex formation, stoichiometric pH titration were performed for the solutions having metal ligand ratio 1:1, 1:2 ,1:3 and 1:4. After each addition of titrant NaOH, pH meter readings were noted on reaching equilibrium. Curves were plotted between pH and moles of alkali required per mole of ligand ‘m’ the inflections in the curves reveal the formation of complexes in solutions from which the stoichiometry has been evaluated.
The stability constants of metal ligand complexes were evaluated by employing Irving Rossotti method10-11 pH titration technique keeping the ratio of metal to ligand constant at 1:5 using 8 x 10-3 M HClO4 for initial lowering of buffer region.
Result and Discussion
The values of formation function n were plotted against the corresponding free ligand concentration (pL) values to get the formation curves of the metal complexation equilibrium. From these formation curves, the values of stability constants, log K1 and log K2 were calculated which correspond to pL values at = 0.5 and 1.5 respectively. The log K stab values were determined at different temperature 20°,30°C and 40°C and at varying ionic strength 0.05M, 0.1M, 0.15M and 0.2M in the pH range 4.25-8.85 are summarized in table 1 and 2 which follow the sequence.
Co2+< Ni2+< Cu2+ > Zn2+
Which is in agreement with Irving William order of stability constants of bivalent metal complexes.
Effect of Temperature and Ionic strength
It is seen from Table 1 that the stability constants gradually increase with rise in temperature showing thereby that higher temperature favours the formation of stabler complexes. The effect of increase in ionic strength from 0.05M to 0.2M on the stability has been examined and it is observed that log K increases with rise in ionic strength. These changes have shown in Table 2.
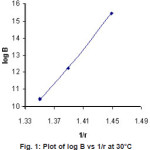 |
Figure 1: Plot of log B vs 1/r at 30°C Click here to View figure |
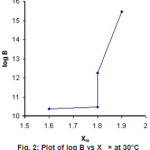 |
Figure 2: Plot of log B vs Xm × at 30°C Click here to View figure |
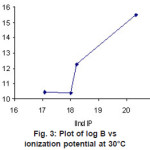 |
Figure 3: Plot of log B vs ionization potential at 30°C Click here to View figure |
Table 1: Stability constant of Co2+, Ni2+, Cu2+ and Zn2+ Complexes of DL-2- Amino-butanedioic acid at varying temperatures (Ionic strength 0.10 M)
| Metal complexes | Log K | 20°C | 30°C | 40°C | |
| Co2+ | Log K | 1 | 5.8631 | 6.0532 | 6.2930 |
| Log K2 | 4.3676 | 4.4188 | 4.5184 | ||
| Log β | 10.2391 | 10.4720 | 10.8114 | ||
| Ni2+ | Log K | 1 | 6.8914 | 7.0914 | 7.2200 |
| Log K2 | 5.0663 | 5.1677 | 5.2305 | ||
| Log β | 11.9577 | 12.2591 | 12.4505 | ||
| Cu2+ | Log K | 1 | 8.4759 | 8.6833 | 8.8501 |
| Log K2 | 6.6564 | 6.7891 | 6.9665 | ||
| Log β | 15.1323 | 15.4724 | 15.8166 | ||
| Zn2+ | Log K | 1 | 5.6955 | 5.9002 | 6.0938 |
| Log K2 | 4.3686 | 4.4905 | 4.5681 | ||
| Log β | 10.0641 | 10.3907 | 10.6619 | ||
Table 2: Stability constant of Co2+, Ni2+, Cu2+ and Zn2+ Complexes of DL-2-Amino-butanedioic acid at varying ionic strengths(Temp.30°C)
| Metal complexes | Log K | 20°C | 30°C | 40°C | |
| Co2+ | Log K | 1 | 5.9785 | 6.0532 | 6.1959 |
| Log K2 | 4.3173 | 4.4188 | 4.4682 | ||
| Log β | 10.2958 | 10.4720 | 10.6641 | ||
| Ni2+ | Log K | 1 | 6.9977 | 7.0914 | 7.1958 |
| Log K2 | 5.1316 | 5.1677 | 5.2339 | ||
| Log β | 12.2930 | 12.2591 | 12.4297 | ||
| Cu2+ | Log K | 1 | 8.5829 | 8.6833 | 8.7822 |
| Log K2 | 6.7177 | 6.7891 | 6.8659 | ||
| Log β | 15.3006 | 15.4724 | 15.6481 | ||
| Zn2+ | Log K | 1 | 5.7925 | 5.9002 | 6.0536 |
| Log K2 | 4.4165 | 4.4905 | 4.5699 | ||
| Log β | 10.2090 | 10.3907 | 10.6235 | ||
Table 3: Thermodynamic function of Co2+, Ni2+, Cu2+ and Zn2+ Complexes of DL-2-Aminobutanedioic acid at 30° C
| Metal complexes | Thermodynamic Function at 30°C (µ= 0.1M) | ||
| − ∆G | ∆H | ∆S | |
| (kcal/mole) | (kcal/mole) | (cal/degree/mole | |
| Co2+ | 14.52 | 12.08 | 87.79 |
| Ni2+ | 16.99 | 12.23 | 96.43 |
| Cu2+ | 21.45 | 14.36 | 118.18 |
| Zn2+ | 14.41 | 12.50 | 88.81 |
The stability constants of these transitional metals have also been interpreted in relation to atomic properties of these metals such as ionic radii (A°) electronegativity (Pauling) and 2nd ionization potential (eV).
Plot of log β against reciprocal of ionic radii of metal ions (Figure1) shows that the ligand forms less stable complexes with Co2+ and Zn2+ and more stable complexes with Cu2+ in comparison to Ni2+ complex.
The plot of Xm (Electronegativity of metal) against log β (Figure2) shows that stability of these metal complexes increases with increasing electronegativity which suggest that the metal ligand bond would be covalent. Similar observation has been reported by Van Uitert14 and Selbin15.
The second ionization potential rises along the transition series and it becomes maximum Cu2+. A correlation of potential and stability was pointed out by Irving Williams13 and Schwarzenbach Ackernmann and Prue16. A similar correlation was observed in the present case of the plots of log β against second ionization potential (Figure 3).
Thermodynamic Parameters
Thermodynamic functions such as free energy(∆ G), enthalpy(∆ H) and entropy (∆ S) accompanying complexation are determined at 30°C with the help of standard equations17 and are summarized in Table 3.
The negative values of ∆G show that the reaction tends to proceed spontaneously. The positive values of enthalpy indicate the endothermic nature of the reaction process in fair agreement with increasing stability suggesting higher temperature favours the chelation process. A positive change in the entropy strongly indicates the complex formation. The very large entropy changes are also justified by considering the greater availability of coordination sites of these metal ions.
To study the bonding nature of complexes, it is necessary to observe the relation between ionic radii of metal ions and thermodynamic parameters ∆G, ∆H and ∆S, by plotting them against reciprocal of ionic radii of divalent transition metals ions, which show somewhat linear relationship. Martell18 has suggested a dependence of linear relationship between ∆S and 1/r, for divalent metals. This indicate the pre-dominant role of electrostatic force involved in ion association process, a fact which is in agreement with Bjerrum’s ion pair concept.
References
- Sharma S.,Saxena, K.K., and Saxena R.S.;Anales De Quimica.,83 B: 125 (1987).
- Sharma S., Saxena, K.K., and Saxena R.S.; J.Indian Chem. Soc. 53: 479 (1986).
- Saxena R.S., and Dhawan S.K.; J. Indian Chem. Soc., 50: 87 (1983)
- Sharma S., Saxena, K.K., and Saxena R.S.; J. Electro. Chem. Soc. India, 37: 267 (1988)
- Sharma S., Saxena K.K., and Saxena R.S.; J. Chem. Soc. Pak., 7: 105 (1985).
- Agarwal Vishakha and Saxena K.K.; U. Scientist Phyl. Sciences, 6: 230 (1994)
- Taquikhan Badar, Vijaya Kumari S., and Murli Mohan K.; Indian J. Chem. 31A: 28 (1992).
- Kanodari M., Mansour H., Gnandaur M.A., and Salah Haman.; J. Electro. Chem. Soc. India, 43(1): 35 (1994).
- Rathore O.P. and Lavale S.C.; Asian J. Chem., 10(3): 613 (1998).
- Irving H., and Rossotti H.S.; J. Chem., Soc. 76: 2904 (1954).
- Irving H., and Rossotti H.S.; J. Chem. Soc., 74: 3397 (1953).
- Irving H. and Williams R.J.P.; J. Chem. Soc., 3192 (1953).
- Irving H., and Williams R.J.P., Nature, 162: 148 (1948).
- Van Uitert, L.G. Ferneteus W.C. and Douglas B.E.; J. Amer. Chem. Soc., 75: 2736 (1953).
- Dey, M.C. and Selbin J.; “Theoritical Inorganic Chemistry”, Reinhold, N.Y. 114 (1957).
- Scharzenbach G., Ackerman H. and Prue J.E.; Nature, 163: 723 (1949).
- Yatrimiriski K.B. and Vasilev V.P.; Instability constant of complex compounds, Pergamon Press Oxford (1960).
- Martell A.E.; Rec. Trav. Chim., 75: 781 (1956).

This work is licensed under a Creative Commons Attribution 4.0 International License.









