Synthesis, structure and physico-chemical studies of Mn (II) complexes of salicylaldehyde derived semicarbazone and thiosemicarbazone
N. K.Sharma1, Sapna1, Manoj Agarwal2, Seema Kohli1, B. Tiwari3, J. N. Gurtu4 and Bhavna5
1Department of Chemistry, M.M.H College Ghaziabad (India).
2Department of Chemistry, K.N.G.D. College of Engineering, Modi Nagar (India).
3Department of Chemistry, Trident College of Engineering, Ghaziabad (India).
4Department of Chemistry, Meerut College, Meerut (India).
5Department of Chemistry, International College of Engineering, Ghaziabad (India).
Complexes of semicarbazone and thiosemicarbazone of salicylaldehyde with Mn(II) have been synthesized by ethanolic solution of 0.05 mole of MnCl2.4H2O and 0.1 mole of corresponding ligand (ie, semi and thiosemciarbazone). Crystal data are reported with variable coordination modes. The complexes have been characterized by various physico-chemical techniques, viz. magnetic susceptibility measurement, electronic, infra-red and electron spin resonance spectral studies. These complexes are found of the type of Mn (ligand)2 X2 {X = Cl-1, Br , So42- and SCN-1 }. All the complexes show a very good agreement with standard magnetic moment value of complexes having five unpaired spins at room temperature They show magnetic moments in the range of 5.93 – 6.03 BM at room temperature. Electronic spectra of the complexes display weak absorption bands in the range 18200-20400 cm-1, 21060-25100 cm-1, 24870-29860 cm-1, 31300-33350 cm-1 which are characteristics of octahedral geometry .
KEYWORDS:Semicarbazone; thiosemicarbazone Ligand; Magnetic susceptibility electronic spectra IR; ESR
Download this article as:| Copy the following to cite this article: Sharma N. K, Sapna, Agarwal M, Kohli S, Tiwari B, Gurtu J. N, Bhavna.Synthesis, structure and physico-chemical studies of Mn (II) complexes of salicylaldehyde derived semicarbazone and thiosemicarbazone. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Sharma N. K, Sapna, Agarwal M, Kohli S, Tiwari B, Gurtu J. N, Bhavna.Synthesis, structure and physico-chemical studies of Mn (II) complexes of salicylaldehyde derived semicarbazone and thiosemicarbazone. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23465 |
Introduction
Semicarbazide (H2N.NHCONH2) is a reagent for identification of aldehyde and ketones and has application in medicine as a lathyrogenic1-5 and a teratogenic6. It form complexes with polynucleotides7 and enhances growth of polio8, rubella viruses9. The possibility for semicarbazide to act as a bidentate ligand has been suggested10. Thiosemicarbazone and their metal complexes have received considerable attention because of their antibacterial,antifungal,antitumor, antiamoebic and antimalarial, antiviral, radio-protective, trypanocidal and anti-inflammatory activities11-22.
Among the various class of biologically active co-ordination compound complex with hetrocyclic thiones as ligands play an important role in biological process and they are used to an effective plant protecting agents23.
Theoretical details
Mode of Coordination
(2a)Bonding in Semicarbazone Complexes
The semicarbazide may act as a monodentate or bidentate chelating agent. Crystal structure of some semicarbazide complex studies and showed that semicarbazide is a bidentate. Crystal structure of some semicarbazide complexes, eg. Cu(Sem)2Cl2 and Zn(Sem)2Cl2 has shown that each metal atom is surrounded by a trans-planar arrangement of two oxygen and twohydrazinic nitrogen which are found at the corners of a distorted square.
Coordination is completed by two chlorine on opposite sites of the square such an octahedral coordination more or less is alikely to be found in the cd complexes and in other disemicarbazide compound as well.24.
(2b)Bonding inThiosemicarbazone complexes
The chemistry of transition metal complexes of thiosemicarbazone has been receiving considerable attention largely because of their pharmacological properties. In the solid state, these thiosemicarbazones exist in the thione form fig (i) In solution however they are known to tautomerize into the thiol fig.(ii) form complexation usually takes place via dissociation of the acidic proton resulting in the formation of a five membered chelate ring fig.(iii) When an additional donor site d is incorporated in such ligands linked to the Carbonylic carbon via one or two intervening atoms, D,N,S tri coordination fig. (iv) usually takes place.25
A very large number of metal complexes involving thiosemicarbazide derivative as ligands have been prepared and studies26-31 including also mixed macrocyclic complexes containing crown ether moieties.
These type of ligands are interesting because of their ability to form hepta-coordinated – bipyramidal (PBP) complexes even with some 3d elements which characteristically do not form such complxes. these planar pentadentate ligands occupy the equatorial plane while the PBP surrounding of the metal is completed with two monodentate ligands at the axial position.
It has been shown33 that the thiosemicarbazide molecule itself exists in the trans-configuration and when complexing in this configuration it behave as a monodentate ligand bonding only through the sulphur atom.
They shown that34 monodentate nature bonding may also occur through the hydrazinic nitrogen in fig (1a) and fig(1b) showing bidentate nature bonding through hydraginic nitrogen and amide nitrogen if any radical is attached with sulphur center.
Experimental
Present Work
Preparation of Complexes
Mn (ligand)2 Cl2
Ethanolic solution of 0.05 mole of MnCl2.4H2O and 0.1 mole of corresponding ligand (ie, semi and thiosemciarbazone) was mixed and then the mixture was refluxed on water bath for 1½ hours. On cooling, light pink to cream coloured complex was separated out. The complex was filtered, washed with ethanol and dried over P4O10.
Mn (ligand)2X2 (X=Br– or SCN–)
Mn Cl2.4H2O (0.05 mole) and KX (X=Br– or SCN– (0.1 mole) were dissolved in 30 ml ethanol. To this 0.1 mole ethanolic solution of corresponding ligand (ie, semi and thiosemicarbazone) was added. The mixture was refluxed on water bath for one hour. On cooling, camel to cream coloured complex was seperated out. The complex was filtered, washed with ethanol and dried over P4O10.
Mn (ligand)2 SO4
Ethanolic solution of 0.05 mole of MnSO4.H2-O and 0.1 mole of corresponding ligand (ie, semi and thiosemicarbazone) was mixed and then the mixture was refluxed on water bath for two hours. On cooling, camel to light pink coloured complex was separated out. The complex was filtered, washed with ethanol and dried over P4O10.
Results and Discussion
All the complexes have composition Mn(ligand)2X2 [X=Cl–, Br–, SO42- and (SCN)–] show a very good agreement with standard magnetic moment value of complexes having five unpaired spins at room temperature as in Table 1.In the high spin octahedrally coordinated Mn2+ complexes, the lowest configuration (t2g)3 (eg)2 gives rise to the ground state 6A1g. Since this is the only sextet level present, all the absorption bands35 musttherefore, bespin forbidden transitions.
Electronic spectra of the complexes display weak absorption bands in the range 18200-20400 cm-1, 21060-25100 cm-1, 24870-29860 cm-1, 31300-33350 cm-1 which are characteristics of octahedral geometry. These bands may be assigned as 6A1g →4A1g (4G) (10B+5C), 6A1g →4Eg (4G) (10B+5C), 6A1g → 4Eg (4D) (17B+5C) and 6A1g → 4T1g (4P) (7B+7C) transitions, respectively (Table 2). The experimentally observed transition energies and calculated values for parameters B,C, Dq and b are shown in Table 3. For the parameter C, the transition 6A1g → 4Eg (4G) and 6A1g → 4T1g (4P), has negative value which has no physical significance, while these transitions yield absurd values for parameter B and hence these values are not included in the table 3. The best set of the value for parameters B and C could be obtained using transitions 6A1g → 4A1g (4G) and 6A1g → 4Eg (4D). The value of Dq were taken from the work of Orgel36. Slater Condon – Shortly parameters F2 and F4 are related to the Racah inter – electronic repulsion parameters B and C, as follows37 B = F2 – 5F4 and C = 35F4. By using values of the Reach parameter B and C, values for the parameter F2 and F4 have been calculated (Table 3).
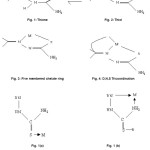 |
Figure 1 – 4 Click here to View figure |
Trans configuration of thiosemicarbazide showing monodentate nature (Figure Ia) Bonding through the sulphur atom only.
(figure Ib) Bonding through the hydraginic nitrogen and amide nitrogen.
Table 1: Magnetic moment (B.M.) and ESR data
| Complexes | µEffec (B.M.) | g |
| Mn (SSC)2Cl2 | 5.87 | 2.014 |
| Mn (SSC)2Br2 | 6.06 | 2.122 |
| Mn (SSC)2SO4 | 5.94 | 2.116 |
| Mn (STSC)2Cl2 | 5.97 | 2.076 |
| Mn (STSC)2Br2 | 6.04 | 2.052 |
| Mn (STSC)2SO4 | 5.99 | 2.021 |
| Mn (STSC)2(SCN)2 | 6.05 | 2.037 |
Table: 2 Electronic spectral bands (cm-1) and their tentative assignment
|
Complexes |
6A 1g→4p 4T 1g |
6A 1g→(4D) 4E g |
6A 1g→(4G 4E g 6A |
1g(4G)→ 4A1g |
|
Mn (SSC)2Cl2 |
33350 |
25102 |
21060 |
19484 |
|
Mn (SSC)2Br2 |
32266 |
29860 |
25100 |
20000 |
|
Mn (SSC)2SO4 |
33202 |
28550 |
25100 |
18200 |
|
Mn (STSC)2Cl2 |
31300 |
28450 |
23605 |
19335 |
|
Mn (STSC)2Br2 |
32254 |
25475 |
24554 |
18632 |
|
Mn (STSC)2SO4 |
31556 |
24870 |
23500 |
18620 |
|
Mn(STSC)2(SCN)2 |
31804 |
29530 |
24502 |
20400 |
Table 3 : Ligand field parameters
| Complex | D (cm-1) q | B(cm-1) | Hx(cm-1) | b | F4 | F2 | C |
| Mn (SSC)2Cl2 | 715 | 690 | 3270 | 0.86 | 95 | 1158 | 2.23 |
| Mn(SSC)2Br2 | 740 | 725 | 3150 | 0.89 | 96 | 1033 | 3.42 |
| Mn (SSC)2SO4 | 760 | 610 | 3168 | 0.79 | 93 | 1068 | 3.64 |
| Mn (STSC)2Cl2 | 687 | 650 | 3358 | 0.82 | 96 | 1136 | 3.06 |
| Mn (STSC)2Br2 | 665 | 648 | 3028 | 0.83 | 89 | 1080 | 3.27 |
| Mn(STSC)2SO4 | 658 | 629 | 3227 | 0.81 | 92 | 1089 | 3.6 |
| Mn(STSC)2(SCN)2 | 734 | 720 | 3415 | 0.92 | 97 | 1206 | 1.54 |
The calculated values of β and Hx of complexes as in Table 3 shows appreciable ionic character. An estimate of β has been obtained from the nephelauxetic parameter, Hx, for the ligand and nephelauxetic parameter, Km, of the metal ion as 1.2 (1 – b) = Hx Km. The values of parameter (Hx) for the complexes have been calculated using covalency contribution of Manganese (II) ion (0.07). The numerical value 786 cm-1 for b of the three Mn2+ ion38 has been used to calculate the value for β.
The ESR spectra of the complexes have been recorded as polycrystalline sample as shown in Fig 1.a – 1.b. Polycrystalline sample gives one broad isotropic signal centered around approximately free electron g – value as in Table I. ESR Signal is easily detected over a large range of temperatures in any crystal field symmetry since there is no nearby crystal field state. Further, a resonance is readily detected even for a large zero-field splitting, because d5 is an odd- electron system whose ground state is a Kramer‘s doublet and whose degeneracy is only completely removed by a magnetic field.
Conclusion
It is concluded that all the complexes were found to have composition Mn(ligand)2X2 (where X=Cl–, Br– and ½ SO42-). They show magnetic moments in the range of 5.93 – 6.03 BM at room temperature. Electronic spectra of the complexes display weak absorption bands in the range 18200 – 20400cm-1, 21060– 2500cm-1,24870 – 29860 cm-1, 31300 – 33350cm-1, assigning 6A1g →4A1g (4G) (10B+5C), 6A1g → 4Eg (4G) (10B+5C), 6A1g → 4Eg (4D) (17B+5C) and 6A1g → 4T1g (4P) (7B+7C) transitions, respectively. A six coordinate octahedral geometry has been assigned for these complexes.
Acknowledgements
The author N Kumar is obliged to Dr M Q Ansari, Department of Chemistry, Multanimal Modi College, Modinagar – 201204 (UP) for valuable discussion and encouragement.
References
- C.I. Levena – Lab – Invest. 19: 25 (1968).
- M Adam, Z Deyl, and J. Rosmus. Med. Farmacol. Exp. 14: 129 (1966).
- H.B. Bensusan, S.D. Mcknight, and M.S.R . Naidu. Biochem. Biophys. Res. Commun. 23: 128 (1966).
- J.J. Lalich. Proc. Soc. Exp. Biol. Med. 123: 214 (1966).
- R. Della Volpe, A. Gandini, and G.M Merli. Biochim. Biol. Sper. 3: 388 (1964).
- F. Bodit, C.R. Soc. Biol. 160: 960 (1966).
- H. Seictel Biochem, Biophys. Acta 129: 412 (1966).
- J. Pages. Ann. Inst. Pasteur 112: 581 (1967).
- A. Vaheri, M.D. Sedwick and S.A. Plotkin, Proc. Soc. Exp. Biol. Med. 125: 1092 (1967).
- K.A. Jensen and E Rancke – Madsen . Z . Anorg. Allgm, Chem. 227: 25 (1936).
- S. Chandra and L.K. Gupta Spectrochimica Acta Part A, 62: 1089-1094 (2005).
- G. Atassi P. Dumnot and J.C. Harteell European Journal of Chemistry 15: 451-459 (1979).
- E.W. Ainscough A.M. Brodie, W.A. Denny, G.j. Finlay and J.D. Ranford (Journal of Inorganic Biochemistry) 70: 175-185 (1998).
- K. Husain A.R. Bhat and A. Azam, European Journal of Medicinal Chemistry 43: 2016-2028 (2008).
- M.C. Rodriguez – Arguelles P. Touron – Touceda R. Cao et al Journal of Inorganic Biochemistry 103: 35-42 (2009).
- W. Seebacher, R. Brun and R. Weis European journal of pharmaceutical sciences 21: 225-233 (2004).
- A. Kolocouris, K. Dimas C. Pannecouque et al., as Bioorganic & Medicinal Chemistry Letters 12: 723-727 (2002).
- G. Aguirre, L. Boiani, H. Cerecetto et al Bioogranic & Medicinal Chemistry 12: 4885-4893 (2004).
- X. Du. C. GuO E. Hansell et al journal of Medicinal chemistry 45: 2695-2707 (2002).
- X. Du, E. Hansell, J.C. Engel, C.R. Caffrey F.E. Cohen and J.H. Mckerrow Chemistry & Biology 7: 733-742 (2000).
- K.S. Abou Melha, Journal of Enzyme Inhibition and Medicinal Chemistry 23: 493-503 (2008).
- S. Chandra and U. Kumar SpectroChimica Acta part. A, 60: 2825-2829 (2004).
- Kukalenko, S,S, Bovykin, B.A., Shesta – Kova, S.I. Omel Chenko, A.M Russian Chemical reviews 54: 1152 (1985).
- Nardelli, M., Gasparri, G.F., Boldrini . P. $ Battistuni, G.G., Acta Crystallography 19: 49 (1965).
- Indrani Pal, falguni Basuli and Samaresh Bhattacharya proc Indian Acad Sci (Chem. Sci) 114(4): 255-268 (2002).
- M.J.M. Campbell, Coord. Chem Rev. 15: 279 (1975).
- S.B. Pandhye, G.B. Kauffman, Coord Chem 63: 127 (1983).
- D.X West S.B. Padhye, P.B. Sonawani Struct. Bond 76(1): (1991).
- J.S. Kasas, M.S. Garcia – Tasende J.J. Sardo Coord. Chem Rev. 209: 197 (2000).
- V. Arion M. Revenko, J Gradianaru Yu Simenov V Kravtsov, N. Gerbeleu, E. Saint – Aman. F. Admas Rev. Inorg. Chem 21(1) (2001).
- V.M. Leovac, V.I. Cesljevic, Koordinaciona hemija, izotiosemikarbazida Injegovin derivata, Mono grafijia prirodno – matematicki fakullet Novi Sud (2002).
- G. Dessy. V. Fares Cryst struct Comm 10: 1025 (1981).
- Domiano, P. Feva, G. Nardeli, M. and Sgara Botio, Acta Crystallogr 25: 343 (1969).
- Gerbeleu, N.V.Revenko, M.D. and Leovals Russ. J. Inorg. Chem. 22: 1009 (1977).
- Heidt C J, Koster G F and Johnson A N, J Am Chem Soc, 80: 6471 (1958 ).
- Orgel I F, J Chem Phys, 23: 1004 (1955).
- Huheey J F, Principles of Structure and Reactivity, Ed. Harper and Row Inst Edition New York, 363 (1972).
- Jorgenson C K, Oxidation mper and Oxidation States Springer New York, 106, (1969).

This work is licensed under a Creative Commons Attribution 4.0 International License.









