Synthesis of Some New 1,3-Thiazolyldiphenyl Amine Derivative and Evaluation of their Antibacterial Effects
S. Singh, V. Kumar, S. K. Sharma, A. Kumar and S. Sha
1Medicinal Chemistry Division, Department of Pharmacology, L.L.R.M. Medical College, Meerut - 250 004 (India).
2Department of Chemistry, D. J. College of Engineering and Technology, Niwari Road, Modinagar - 201 204 (India).
In a search for new leads towards potent antibacterial agents, an array of novel N-[2-(3
KEYWORDS:Diphenylamine;` thiazole; thaiazolidinone; azetidinone; antibacterial; Inhibitory zone diameter; Minimal inhibitory concentration
Download this article as:| Copy the following to cite this article: Singh S, Kumar V, Sharma S. K, Kumar A, Sha S. Synthesis of Some New 1,3-Thiazolyldiphenyl Amine Derivative and Evaluation of their Antibacterial Effects. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Singh S, Kumar V, Sharma S. K, Kumar A, Sha S. Synthesis of Some New 1,3-Thiazolyldiphenyl Amine Derivative and Evaluation of their Antibacterial Effects. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23455 |
Introduction
In recent decades, problems of multi-drug resistant microorganisms have reached on alarming level in many countries around the world, and also infections caused by those microorganisms pose a serious challenge to the medical community and the need for an effective therapy has led to a search for novel antibacterial agents. The development of bacterial resistance of existing drugs is a major problem in antibacterial and necessitates continuing research in to new classes of antibacterial1. Thiazole consists a unique class of five member rings containing nitrogen and sulphur hetrocycles there has been considerable interest in thiazole ring system, with regard to heterocyclic chemistry and pharmacological activities of several of its derivatives. Thiazole and its derivatives have been studied extensively because of ready accessibility, diverse chemical reactivity, and broad spectrum of biological activities such as anti inflammatory2, 3, antiviral4, 5, antifungal6, 7, antibacterial8, 9 and many more. Furthermore, derivative of schiff base10, 11, azetidinone12, 13, thiazolidinone14, 15 have also been found to possess promising antibacterial activity. In view of these observations, we thought that it would be interesting to synthesize the substituted derivatives starting form diphenylamine followed by combination of thiazolyl azetidinone or thiazolylthiazolidinone in one frame may lead to compounds with interesting antibacterial profile.
In the present work, N-(chloroacetyl) diphenylamine (1) has been obtained from condensation of chloroacetyl chloride in dioxane. Compound 1 is then converted in to N-(2-amino-1,3-thiazol-4-yl)diphenylamine (2) reaction with thiourea. The reaction of compound 2 with aromatic aldehydes in presence of glacial acetic acid gave the Schiff bases: N-(2-substituted benzylidenylimino-1,3-thiazol-4-yl)diphenylamine 3(a-e). Compounds 3(a-e) were reacted with triethyl amine and chloroacetyl chloride to give their azetidinone congers. i.e. N-[2-(3´-chloro-2´-oxo-4´-substituted aryl-1-azetidinyl)-1,3-thiazol-4-yl]diphenylamine 4(a-e). On the other hand, reaction of compounds 3(a-e) with thioglycolic acid in the presence of anhydrous zinc chloride led to the formation of N-[2-(2´-substitutedaryl-4´-oxo-1´,3´-thiazolidin-3´-yl)-1,3-thiazol-4 yl)diphenylamine 5(a-e). The structures of newly synthesized compounds were delineated by elemental (C, H, N) and spectral (IR, 1H-NMR, Mass) data. The synthetic route ofabove said compounds is outlined in Scheme I.
Material and Methods
Chemistry
All the reagents and solvents were generally received form commercial supplier. Reactions were done in dried glassware. Melting points were taken in open capillaries by thermonic melting point apparatus, and are uncorrected. The purity of the newly synthesized compounds was checked by thin layer chromatography (TLC) on silica gel-G coated plates by using different solvent systems. Infrared (IR) spectra were determined on Bruker IFS-66 FTIR (Bioscience, USA) using KBr pallets and wave number (í) was reported in cm-1. The 1H-NMR spectra were taken on Jeol GSX -300 FT NMR (Jeol, Tokyo, Japan) in CDCl3 or DMSO-d6, and chemical shifts (δ) are given in ppm. Tetramethylsilane (TMS) was used as internal reference standard. Mass spectra were recorded on Spec Finnigan Mat 8230 MS (Thermo Electron Corporation). The carbon, hydrogen and nitrogen analysis were performed on Carlo Erba-1108 (Carlo Ebra, Milan, Italy), and the results were found within + 0.4% of the theoretical values.
General procedure for synthesis of N-(chloroacetyl) diphenylamine (1)
A solution of chloroacetyl chloride (0.02 mol), in dry dioxane (200 ml), was added to the solution of diphenylamine (0.01 mol) in dry benzene (50 ml) at 0-5 oC temperature with constant stirring during half an hour. Then, the reaction mixture was refluxed for 4 h, then cooled and poured onto a mixture of water (300 ml) and ether (100 ml). The resulting mixture was filtered to get solid, which was crystallized from ethanol to give compound 1: m.p 108 oC, yield 65%, Anal. C14H12NOCl; requires: C, 68.43; H, 4.89; N, 5.70. Found: C, 68.55; H, 4.78; N, 5.90. IR (KBr) n 3075 (C-H aromatic), 2964 (C-H aliphatic), 1706 (C=O), 1572 (C—-C of aromatic ring), 1543 (C-N-C), 710 (C-Cl) cm-1. 1H-NMR (CDCl3) δ: 7.42-7.83 (m, 10H, Ar-H), 3.39 (s, 2H, CH2Cl) ppm. MS: m/z 245.5 (M+), 247.5 (M+2).
General procedure for synthesis of N-(2-amino-1, 3-thiazol-4-yl)diphenylamine (2)
To a solution of compound 1 i.e. N-(chloroacetyl) diphenylamine (0.01 mol) in ethanol (150 ml), thiourea (0.01 mol) was added. This reaction mixture was heated under reflux for 12-15 h with occasional stirring. Then, the reaction mixture was concentrated, and the residue obtained was poured over crushed ice and then crystallized from methyl alcohol and ethyl acetate. The base was liberated by dissolving the hydrochloride in water and basifying with a saturated solution of sodium carbonate and with the water to liberate the base completely, dried and crystallized from absolute ethanol to give compound 2: m.p. 117 oC, yield 55%, Anal. C15H13N3S; requires: C, 67.42; H, 4.87; N, 15.73. Found: C, 67.33; H, 4.75; N, 15.64. IR (KBr) n 3265 (NH2), 3075 (C-H aromatic), 2964 (C-H aliphatic), 1595 (C=N), 1564 (C—-C of aromatic ring), 1543 (C-N-C), 685 (C-S-C) cm-1. 1H-NMR (DMSO-d6) δ: 7.40-7.84 (m, 10H, Ar-H), 6.20 (s, 2H, NH2, exchangeable with D2O), 5.23 (s, 1H, CH of thiazole) ppm. MS: m/z 267 (M+)
General procedure for synthesis of N-(2-substitutedbenzylidenylimino-1,3-thiazol-4-yl) diphenylamine 3(a-e)
The equimolar amount of compound 2 (0.02 mol) and different aromatic aldehydes (0.02 mol) in methanol (55 mL) was refluxed for 10-12 h in presence of few drops of glacial acetic acid. The progress and completion of the reaction were checked by TLC. After refluxing, excess of solvent was distilled off and remnant was dropped on crushed ice, filtered, dried and solids thus obtained were recrystallized from appropriate solvents to furnish compounds 3(a-e). Their characterization data and spectral data are given in Table 1 and 2, respectively.
General procedure for synthesis of N-[2-(3´-chloro-2´-oxo-4´-substituted aryl-1-azetidinyl)-1, 3-thiazole-4-yl]diphenylamine 4(a-e)
To the different solutions of compounds 3(a-e) (0.01 mol) in dry dioxane (50 mL), chloroacetyl chloride (0.02 mol) and triethyl amine (0.01 mol) were added with stirring at 0-5 °C temperature. These different reaction mixtures were, further, refluxed for 4 to 6 h and excess of solvent then distilled off. The resultant mixtures were poured onto crushed ice to afford compounds 4(a-e). The physical and analytical data of these compounds are given in Table 1, and spectral data are furnished in Table 2.
General procedure for synthesis of N-[2-(2´-substituted aryl-4´-oxo-1´, 3´-thiazolidin-3´-yl)-1, 3 thiazol-4-yl]diphenylamine 5(a-e)
To the separate solutions of compounds 3(a-e) (0.02 mol) in DMF (50 ml), thioglycolic acid (0.02 ml) and anhydrous ZnCl2 (0.02 mol) were added. These reaction mixtures were heated under reflex for 6 h. After completion of reaction, excess of solvent was distilled off, then cooled, and poured onto crushed ice. Solids thus separated out were crystallized from appropriate solvent to furnish compounds 5(a-e). The physical and analytical data are shown in Table 1 and spectral data present in Table 2.
Pharmacological Evaluation
The compounds 3(a-e), 4(a-e) and 5(a-e) and standard drug, amphicillin, have been evaluated for antibacterial activity. In order to determine the antibacterial activity of proposed compounds, minimal inhibitory concentration (MIC) and inhibitory zone diameter were tested against Staphylococcus aureus ATCC 25923 (S. aureus), Bacillus Subtilis ATCC 6051 (B. Subtilis) and Staphylococcus epidermis ATCC 14940 (S. epidermis) and gram negative bacteria Escherichia Coli ATCC 25922 (E. Coli), Klebsiella pneumoniae ATCC 10031 (K.pneumoniae) and Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa).
Antibacterial activity
The newly synthesized compounds and reference drug were screened for antibacterial activity against different bacterial strains at a concentration of 250 µg/ml by filter paper disc method16. Results of the study are shown in Table 3. DMSO served as control and due this there was no visible change in bacterial growth. The discs of Whatmann filter paper were prepared with standard size (7 mm) and kept into 1 Oz screw capped wide mouthed containers for sterilization. These bottles are kept in to hot air oven at 150 oC. Now, solution is then put into each bottle. The discs are transferred to the inoculated plates with a pair of fine pointed tweezers. To prevent contamination tweezers may be kept with their tips in 70% alcohol and flamed off before use. Before use the test organism, which were grown on nutrient agar. They were sub cultured in nutrient broth at 37 oC for 18-20 h. Carefully each disc was applied to the surface of agar without lateral movement once the surface had been touched. Now the plates incubated for 24 h at 37 oC.
Minimal inhibitory concentration (MIC)
The antimicrobial activity was assayed in vitro by the twofold broth dilution17 against different bacterial stains and results are depicted in Table 3. The minimal inhibitory concentrations (MIC, µg/ml) were defined as the lowest concentrations of compound that completely inhibited the growth of each strain. All compounds, dissolved in dimethylsulfoxide, were added to culture media.Mueller Hinton Broth for bacteria to obtain final concentrations ranging from 100 µg/ml to 0.781 µg/ ml. The amount of dimethylsulfoxide never exceeded 1% v/v. Inocula consisted of 5.0 × 104 bacteria/ml and 1.0 ×103 fungi/ml. The MICs were read after incubation at 37 °C for 24 h. Media and media with 1% v/v dimethylsulfoxide were employed as growth controls.
Results and Discussion
The antibacterial screening showed that all the tested compounds 3(a-e), 4(a-e) and 5(a-e) showed moderate to excellent inhibitory growth against gram positive bacteria S. aureus, B. Subtilis and S. epidermis and gram negative bacteria E. Coli, K. pneumoniae and P. aeruginosa at 250 µg/ml concentration using standard method.
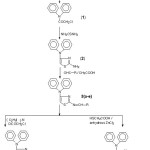 |
Scheme 1 Click here to View Scheme |
 |
Table 1: Characterization data of compounds 3(a-e), 4(a-e) and 5(a-e) Click here to View table |
Compound 4a having o-hydroxyphenyl substitutent exhibited excellent antibacterial activity against S. aureus with MIC 3.125 µg/ml. Compound 5b having p-methoxyphenyl group as substitutent more potent antibacterial activity against S. aureus and S. epidermis with MIC 3.125 µg/ml. Compound 4b and 4e bearing β-lactam ring posses p-methoxyphenyl and p-aminodimethylphenyl group as substitutent, respectively revealed excellent antibacterial activity against all bacterial strains with MIC 0.781- 6.25 µg/ml. Compound 5e bearing thiolactum ring also show adequate antibacterial activity against all bacterial strains with MIC 0.781-6.25 µg/ml as compared to standard drug.
From the results, it is found that compound 4a, 4d, 5a and 5d reflected significant antibacterial activity against gram positive bacteria and gram negative bacteria as compared to standard drug. Rest compounds of this series were least potent than reference drug.
The antibacterial result depicted in Tables 3 indicated that the conversion of compounds 3(a-e) into their corresponding azetidinone congeners 4(a-e) and thiazolidinone congeners 5(a-e) increases the inhibition action against the growth of different bacterial strains. However, compound 4(a-e) bearing β-lactam ring exhibited better antibacterial activity as compared to thiazolidinone ring bearing compounds 5(a-e). by examining the effects of different substituting group o-hydroxyphenyl, p-methoxyphenyl, phenyl, p-hydroxyphenyl, p-aminodimethylphenyl Furthermore, among azetidinone and thiazolidinone congeners, compounds having p-methoxyphenyl group (4b, 5b) and p–aminodimethylphenyl (4e, 5e) showed better activity in their respective groups against different gram positive and gram negative bacterial strains.
At the end, it may be concluded that-
- Presence of p-methoxyphenyl and p-aminodimethylphenyl as a substituent elicits a remarkable increase in biological profile.
- Cylcylization of substituted schiff bases into their corresponding azetidinone and thiazolidinone congeners enhances the antibacterial activity
- Compounds having azetidinone moiety displayed better biological results than those containing thiazolidinone ring.
 |
Table 2: Spectral data of compounds 3(a-e), 4(a-e) and 5(a-e) Click here to View table |
 |
Table 3: Antibacterial data of compounds 3(a-e), 4(a-e) and 5(a-e) Click here to View table |
Acknowledgements
The authors are thankful to Sophisticated Analytical Instrument Facility, Indian Institute of Technology Madras, Chennai, India for spectral and elemental analysis and Head, Department of Microbiology, L.L.R.M. Medical College, Meerut, India for antifungal and antibacterial activities. The authors have declared no conflict of interest.
References
- Reck F., Zhou F., Girardor M., Kern G., Eyermann C. J., Hales N. J., Ramsay H. H. and Granestock M. B., J. Med. Chem., 8: 499(2005).
- Kalkhambkar R. G., Kulkarni G. M., Shivkumar H. and Rao R. N., Eur. J. Med. Chem., 2: 1272 (2009).
- Sharma R. N., Xavier F. P., Vasu K. K., Chaturvedi S. C. and Pancholi S. S., J. Enzyme Inhib. Med. Chem., 24: 890 (2009).
- El-Sabbagh O. I., Baraka Mohamed M., Ibrahim S. M., Pannecouque C., Andrei G., Snoeck R., Balzarini J. and Rashad A. A., Eur. J. Med. Chem., 44: 3746 (2009).
- Srivastava P. C., Pickering M. V., Allen L. B., Streeter D. G., Campbell M. T., Witkowski J. T., Sidwell R. W. and Robins R. K., J. Med. Chem., 20, 256(1977).
- Ghorab M. M. and El-Batal A. I., Boll. Chim. Farm., 141: 110(2002).
- Narayana B., Vijaya Raj K. K., Asthalayha B. V., and Suchita Kumari N., Indian J. Chem., 45B: 1704(2006).
- Karegoudar P., Karthikeyen M. S., Prasad D. J., Mahalinga M., Holla B. S. and Kumari S. K., Eur. J. Med. Chem., 43: 261 (2008).
- Naik B. and Desai K. R., Indian J. Chem., 45B: 267(2006).
- Raman N., Thalamuthu S., Dhaveethu J., Raja H., Neelakandan M. A. and Banerjee S., J. Chil. Chem. Soc., 53: 1450(2008).
- Bonsignore L., De Logu A., Loy G., Lavagna S. M. and Secci D., Eur. J. Med. Chem., 29: 479(1994).
- Thaker M. K., Kachnadia V. V. and Joshi S. H., Indian J. Chem., 42B: 1544(2003).
- Sharma P., Kumar A. and Sharma S. Bisheterocyclic synthesis and antimicrobial studies on some biologically significant 2-[N-(3′-chloro-4′-substituted azetidinene-2)]amino-4-hydroxypurines. Indian J. Chem. 43B(2): 585 (2004).
- Vicini P., Geronikaki A., Incerti M., Zani F., Dearden J. and Hewitt M., Bioorg. Med. Chem., 16: 3714 (2008).
- Singh T., Sharma S., Srivastava V. K. and Kumar A., Indian J. Chem., 44B: 1557 (2006).
- Gould J. C. and Bowie J. H., Edi. Med. J., 59: 178(1952).
- Jorgersen J. H., Turnidge J. D. and Washington J. A.. Manual of Clinical Microbiology, American Society for Microbiology, Washington DC, 1275 (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









