Synthesis of Pyrazolone Derivatives and their Biological Activities
C. G. Naik and G. M. Malik*
Department of Chemistry, Navyug Science College, Surat - 395 001 (India).
3-Methyl-1-phenylpyrazol-5-one and its derivatives prepared using ethyl acetoacetate, phenyl hydrazine and its derivatives. Ethyl acetoacetate reacted with phenylhydrazine at reflux temperature to give 3-methyl-1-phenylpyrazol-5-one crude and recrystlized from diluted ethanol to give pure 3-methyl-1-phenylpyrazol-5-one. Different pyrazolone derivatives were prepared by using different phenyl hydrazine derivatives. 3-methyl-1-phenylpyrazol-5-methylemine and its derivatives were prepared by 3-methyl-1-phenylpyrazol-5-one condensed with methyl amine by using methanol as media. The structures of the compounds were also elucidated. The synthesized compounds were found to have significant effect against the tested microorganisms.
KEYWORDS:Pyrazolone. antibacterial and antifungal activites
Download this article as:| Copy the following to cite this article: Naik C. G, Malik G. M. Synthesis of Pyrazolone Derivatives and their Biological Activities. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Naik C. G, Malik G. M. Synthesis of Pyrazolone Derivatives and their Biological Activities. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23478 |
Introduction
An intensive literature survey including the methods of synthesis for various pyrazolone derivatives has been carried out, as the derivatives of pyrazolone have been of interest to medicinal chemists for their wide range of biological activity1. Using the scheme mentioned here pyrazolone derivatives of 5-imine and 5-imine pyrazolone derivatives were synthesized The presence of C=O group is of great importance, considering the fact that it can be transformed into various chloride compounds and imine compounds. The availability of the present and significant biological properties of the members known so far prompted to extend moieties like ether linkages and imine to amine derivatives. In this context, the synthesis of pyrazolones and imines of pyrazolone was carried out.
Here the synthesis and IR characterization of some schiff bases from 3-methyl-1-R pyrazol-5-one have been reported. Applications of α-ketoimines as potential extraction and spectrophotometric reagents2 and in the synthesis of interesting tetradentatate β-ketoimine complexes with metals 3-5 have been reported. Some workers have reported the synthesis of some β -ketoimines which they characterized in solution as bidentate and tridentate6-8 ligands, including some schiff bases synthesized from 3-methyl-1-R pyrazol-5-one 9.
Experimental
Melting points were determined by open capillary tube in paraffin, melting point bath and therefore the values reported are uncorrected .The purity of the compounds was checked by TLC, was run on silica gel 60 F254 aluminium sheet using chloroform, ethyl acetate, hexane, toluene, methanol, as developing solvent. IR spectra were recorded on Shimadzu 1700.The IR spectra of the compounds were recorded in the region, 4000 cm-1 -400 cm-1using KBr discs on Shimadzu FTIR 840 OS.
Preparation of 3-methyl-1-phenylpyrazol-5-methyl imine
Mixture of 3-methyl-1-phenylpyrazol-5-one(one mole), methyl amine in 40% in water (five mole) in methanol was refluxed till the reaction completed, to check the progress TLC was checked every two hours. After the completion, reaction mixture was cooled to room temperature gradually then 10 times water added in to it and neutralized with diluted HCl. The product was isolated and crystallized from water.
Preparation of 3-methyl-pyrazol-5-methyl imine. Mixture of 3-methyl-pyrazol-5-one(one mole), methyl amine in 40% in water (five mole) in methanol was refluxed for about ten hrs. Then TLC was carried in suitable solvent system for preparation complied. Added 10 times water in it and neutralized with diluted HCl. The product was isolated and crystallized from water.
Preparation of 3-methyl-1( m – chloro )-phenylpyrazol-5-methyl imine
Mixture of 3-methyl-1(m-chloro)-phenylpyrazol-5- one (one mole), methyl amine in 40% in water (five mole) in methanol was refluxed for twelve hrs. The reaction mixture was slowly cooled to room temperature then TLC was carried in suitable solvent system for preparation complied, added 10 times water in it and neutralized with diluted HCl. The product was isolated and crystallized from water.
Preparation of 3-methyl-1( p – tolyl )-phenylpyrazol-5-methyl imine
Mixture of 3-methyl-1(p-tolyl)-phenylpyrazol-5- one (one mole), methyl amine in 40% in water (five mole) in methanol was refluxed for seven hrs. Then TLC was carried in suitable solvent system for preparation complied, added 10 times water in it and neutralized with diluted HCl. The product was isolated and crystallized from water.
Preparation of antibacterial and antifungal activity
In the agar diffusion method different concentrations are incorporated in to an agar medium. A replicator device may be used to inoculate multiple specimens on to a series of plates with varying concentration of antibiotic. In current study, the antimicrobial activity was carried out by the agar diffussion method. Here responses of organism to the synthesized compounds were measured and compared with the response of the standard reference drug. The standard reference drugs used in the present work were Ampicillin ( antibacterial ) and Amphoterecin-B (antifungal). The plates were incubated at room temperature for 1 hour and then in bacteriological incubators at 37°c for 24 hrs for bacterial and in BOD incubator at 22°c for 24 to 48 hours for fungal, after addition of drugs in the well, which was prepared by sterile cork borer. After incubation the zones were measured with the help of digital zone reader and were compared with standard drugs.
IR spectra
The characterization absorption peaks were observed for all relevant groups. The absorption peaks around 1720 cm-1 indicated the formation of imine, (1,3 sub) benzene indicated at cm-1, chloro indicated at 778 cm-1, (1,4 sub) benzene indicated at 818 cm-1, aromatic methyl indicated at 1372 – 1243cm-1, benzene indicated at 756 cm-1, methyl amine indicated at 2921 cm-1.
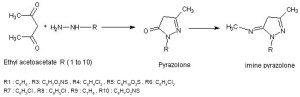
R1 : C6H6 , R3: C6H7O2NS , R4: C6H4Cl2 , R5: C8H10O3S , R6: C6H4Cl2
R7 : C6H5Cl , R8 : C6H5Cl , R9 : C7H8 , R10 : C6H7O2NS
Antibacterial screening
All synthesized compounds were evaluated for in vitro antibacterial activity against four pathogenic organisms by standard agar diffusion method. The zone of inhibition was determined using amicillin as reference standard. All pyrazolone derivatives showed significant antibacterial activity against tested pathogens. However, none of the synthesized compounds were superior to the standard Ampicillin. All the compounds were not active against E-coli.
Antifungal screening
All synthesized compounds were evaluated for in vitro antifungal activity against two pathogenic fungi by standard agar diffusion method. The zone of inhibition was determined using Amphoterecin-B as reference standard. All pyrazolone derivatives did not show significant antifungal activity against tested pathogens. All the compounds were not active against S.cervisiae.
Table 1: Physical data of the synthesised compound
|
S. No. |
Compound Code |
% Yield |
Melting Point |
Mol. Formula |
M.Wt. |
% Calculated |
||
|
C |
H |
N |
||||||
|
1 |
V-1 |
64 |
112-116°C |
C11H13N3 |
187 |
7.58 |
6.95 |
22.46 |
|
2 |
V-2 |
90 |
>200°C |
C5H9N3 |
111.1 |
54 |
8.1 |
37.8 |
|
3 |
V-3 |
80 |
176.5-179°C |
C11H14O2N4S |
166.28 |
49.57 |
5.25 |
21.03 |
|
4 |
V-4 |
80 |
116-120°C |
C11H11N3Cl2 |
256 |
51.56 |
4.29 |
16.4 |
|
5 |
V-5 |
50 |
181-185°C |
C13H17N3S |
295 |
52.88 |
5.76 |
14.23 |
|
6 |
V-6 |
58 |
>200°C |
C11H11N3Cl2 |
256 |
51.56 |
4.29 |
16.4 |
|
7 |
V-7 |
55 |
98-102°C |
C11H12N3Cl |
221.7 |
59.63 |
5.41 |
18.94 |
|
8 |
V-8 |
55 |
90-95°C |
C11H11N3Cl2 |
221.7 |
59.63 |
5.41 |
18.94 |
|
9 |
V-9 |
70 |
100-105°C |
C12H15N3 |
201 |
71.64 |
7.46 |
20.89 |
|
10 |
V-10 |
50 |
171-173°C |
C11H14O2N4S |
266.28 |
49.25 |
5.25 |
21.03 |
Table 2: Antimicrobial screeining-zone of inhibition (mm)
| Microorganism (Bacterial) | V-1 | V-2 | V-4 | V-5 | V-6 | V-7 | V-8 | V-9 | V-10 | Standard Ampicillin |
| S. aureus (mm) | 12.7 | – | 14.9 | 11.5 | – | 12.1 | 9 | – | – | 19.2 |
| B.pumillus (mm) | 12.2 | 11.9 | 13.9 | – | – | 16.5 | 11.9 | – | – | 18.5 |
| E-coli (mm) | – | – | – | – | – | – | – | – | – | 17.3 |
| Ps.Aeruginosa (mm) | – | – | – | 17 | – | – | – | – | – | 13.7 |
| Microorganism (fungal) | V-1 | V-2 | V-4 | V-5 | V-6 | V-7 | V-8 | V-9 | V-10 | Standard Amphoterecin -B |
| C.albicans | – | – | 12.5 | 13 | – | – | 20.2 | – | – | 16.1 |
| S.cervisiae | – | – | – | – | – | – | – | – | – | 15.4 |
References
- R A Pawar and A A Patil , Indian J Chem, 33B, 156-158,( 1994).
- Okafor E C, Talanta, 27: 887 (1980).
- Belcher R , Blessel K , Cardwell T , Praica M , Stephen W I & Uden P C , J Inorg Nucl Chem, 35: 1127 (1973).
- Rao S N , Jaiswal M N , Mishra D D , M aurya R C & Rao N N , Polyhedron, 12, 2945,(1993).
- Holm R H , Everett G W & Chakravorty A s, Prog Inorg Chem, 7: 83 (1966).
- Donia A M & EI – Saied F A , Polyhedron, 2149 (1988).
- EI – Saied F A , Inorg Chim Acta , 165: 147 (1989).
- Kuncheria B and Indrasenan P, Polyhedron , 7: 143, (1988).
- Rao S N , Mishra D D , Maurya R C & Rao N N , Bull Chem Soc Japan , 68: 1589 (1995), Jensen B S, Acta Chem Scand, 13: 1668 (1959).

This work is licensed under a Creative Commons Attribution 4.0 International License.









