Synthesis, Characterization and Biological Activities of Some New Acid Hydrazones Derived from Ethyl-2-(2, 5-Dichloroanilido) Acetohydrazide
Raj N. Sharma, K.P. Sharma, S.N. Dixit and R.L. Taigar
Chemical Laboratories, Government SMS Science College, Gwalior - 474 002 (India).
A series of new acid hydrazones have been synthesized by the reaction of ethyl-2-(2, 5-dichloroanilido) acetohydrazide with various carbonyl compounds in 27 to 92 % yield. Hydrazones are white, brown and yellow colour solids, having high melting points. Newly synthesized compounds (1, 2, 3, 4, 5, 6, 7, 8, 9, 12, 13, 14, 15, 16 and 17) have been tested for their antibacterial activity against gram positive bacteria S.albus, S.aureus and gram negative bacteria E. coli and Pseudomonas piosineus. The compound 2, 3, 5, 12, 13, 14, and 15 shown significant activity and compound 1, 4, 6, 7, 8, 9, 16 and 17 have shown moderate activity. The same compounds were tested for their antifungal activity against Candida albicans, Aspergillus niger and Alternaria alternata at concentration of 30 mg/mL using savored dextrose agar media. The compound 2, 5, 12, 13, 14, and 15 shown significant activities and compound 1, 4, 8, 9, 16 and 17 have shown moderate activity against Candida albicans and Aspergillus niger. All the other compounds did not show significant activity against the fungi at the concentration used. Some new compounds have been tested for anti tubercular activity in-vitro using Mycobacterium tuberculosis. The compounds were incorporated into Lowenstein Jensen egg medium having concentrations of 10 and 100 mg/mL and were inoculated with Mycobacterium tuberculosis, H27, Rv strains, incubated at 370C and observed, the compound Ethyl-2-(2,5-dichloroanilido) acetohydrazide, Ethyl-2-(2, 5-dichloroanilido) acetohydrazone of 4-N,N-Bis -2’- cyanoethylamino benzaldehyde, Ethyl-2-(2,5-dichloroanilido) acetohydrazone of 2-methyl-4-N,N-Bis-2’- cyanoethylaminobenzaldehyde and Ethyl-2-(2, 5-dichloroanilido) acetohydrazone of 5-Chloro Salicylaldehyde inhibited the growth of Mycobacterium tuberculosis at 100mg/mL concentration other compounds were found to be inactive.
KEYWORDS:Malonicester; acidhydrazide; acidhydrazones; synthesis; characterization and biological activities
Download this article as:| Copy the following to cite this article: Sharma R. N, Sharma K. P, Dixit S. N, Taigar R. L, Synthesis, Characterization and Biological Activities of Some New Acid Hydrazones Derived from Ethyl-2-(2, 5-Dichloroanilido) Acetohydrazide. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Sharma R. N, Sharma K. P, Dixit S. N, Taigar R. L, Synthesis, Characterization and Biological Activities of Some New Acid Hydrazones Derived from Ethyl-2-(2, 5-Dichloroanilido) Acetohydrazide. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23568 |
Introduction
Hydrazones possessing an azometine – NHN=CH- Proton constitute an important class of compounds for new drug development. Therefore, many researchers have synthesized these compounds as target structures and evaluated their biological activities. Acid hydrazides have frequently been investigated for testing their potentiality as tuberculostats1-8. Hydrazides and their condensation products have displayed diverse range of biological properties such as bacteriocidal9-10, anti-fungal11, anti-convulsant12-15, anti-helmintic16, anti-tumor17-20, anti-leprotic21, anti-malerial22-23, anti-cancer24-31, anti-depressant32, anti-HIV33, analgesic-anti-inflammatory34, leishmanicidal35, vasodilator activities36.
Experimental
All chemicals used were of A.R. grade (either of B.D.H. or Excel-R or extra pure E. Merk quality). The structure of the compounds were determined by elemental analysis, IR and NMR spectral data. IR spectra (KBr) are recorded on a perkin-Elmer 283 spectrophotometer. NMR spectra (CDCl3) are recorded on varian EM 360 L spectrophotometer. Melting point of the compounds are determined in open capillary tubes and are uncorrected. Purity of the compounds is checked on T.L.C. using silica gel-G. Elemental analysis is performed on Carlo-Erba 1108 analyser.
General procedure
Preparation of Ethyl-2-(2,3-dichloroanilido) ethanoate [1]
A mixture of 2,3-dichloro aniline (5 ml) and diethyl malonate (10ml) was refluxed for 50-55 minutes in a 100 ml r.b. flask fitted with an air condenser of such a length (14²) that ethanol formed escaped and diethyl malonate flowed back in to the flask. Contents were cooled, 30 ml ethanol was added and kept over night, well precipitate found. It was filter under suction and purified by recrystalisation from ethanol.
IR Absorption band (cm–1)
3150 (N–H stretching), 1665–1660 (C=O Ketone), 1090 (C–Cl Stretching), 760–755 (Di substituted benzene), NMR spectra (d Me2CO), 1.1– 1.2 (3 H, t, CH3), 2.21 (2 H, s, CH2), 4.0–4.22 (2 H, q, CH2), 7.0–7.21 (4 H, m, ArH).
Preparation of Ethyl-2-(2,3-dichloro anilido) acetohydrazide [2]
Ethyl-2-(2,3-dichloroanilido) ethanoate [.02 mol (5.52 gm)] was dissolved in rectified sprit in a small 3 neck r.b. flask kept on ice bath and set-up mechanical stirrer. Hydrazine hydrate (80%, 13 ml) was added by dropping funnel slowly drop by drop.
The contents were stirred for 15-20 minutes. There were evolution of heat and reaction was spontaneous after 20 minutes, solid was filtered under suction and recrystallised from ethanol, then we get silver white crystals in good yield.
IR Absorption band (cm–1)
3160 (N–H stretching), 1660 (C=O Ketone), 1595, 1520, 1450 (C=C ring stretching), 760–755 (2, 3 di substituted benzene), NMR spectra (d DMSO), 2.25 (2 H, s, CH2), 3.15 (3 H, s, CH3), 4.12–4.31 (1 H, t, NH), 6.95–7.2 (3 H, m, ArH).
Synthesis of new Acidhydrazones [3]
Ethyl-2-(2,3-dichloroanilido)acetohydrazide (.001 mol) and (.001 mol) of aromatic aldehyde or ketone dissolve in absolute alcohol and added 2-drops of conc. H2SO4 and stirred for 15 minutes. It was filtered under suction and recrystallised from hot ethanol. Synthetic strategy has been out lined in scheme I,II&III. Mechanism for the formation of acid hydrazones is given in chart-I.
IR Absorption band (cm–1)
3150 (N–H stretching), 2960–2970 (C–H aliphatic), 1662–1660 (C=O Ketone), 785–778 (C– Cl Stretching), 760 (2,3-disubstituted benzene), NMR spectra (d DMSO), 2.25 (2 H, s, CH2), 4.21 (1 H, s, NH), 6.95–7.2 (10 H, m, ArH).
Biological evaluation
Anti-bacterial activity
Ten new acid hydrazones (1,3,4,7,8,9,12,13,15 and 16) were screened for their anti-bacterial activity against the gram positive bacteria S. albus, S. aureus and gram negative bacteria E. coli and pseudomonas piosineus using cup-plat agar diffusion technique13 at 10,15,25 mg/ ml concentrations. Maximum inhibition (13-14 mm) was found in 12,13 and 15 against S. albus. Compounds 4,7,8,9 showed moderate activity against S. aureus. No significant activity was displayed by other compounds.
Anti-fungal activity
The same compounds were tested for their anti-fungal activity against candida albicans, aspergillus niger and alternaria alternate at concentration of 30 mg/ml using sabouraud dextrose agar media. Compounds 12,13 and 15 were found to be moderately active against candida albicans and aspergillus niger. All the other compounds did not show significant activity against the fungi at the concentration used.
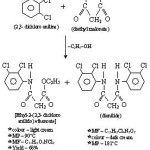 |
Scheme 1 Click here to View Scheme |
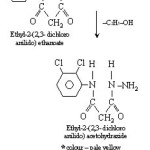 |
Scheme 2 Click here to View Scheme |
 |
Table 1: Physical and analytical data of new compounds: Acid hydrazones derived from 2-(2,3 dichloroanilido) acetohydrazide Click here to View table |
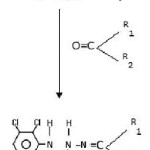 |
Scheme 3 Click here to View Scheme |
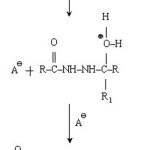 |
Chart 1: [Mechanism of formation of new acid hydrazones] Click here to View Chart |
Acknowledgements
The authors are thankful to Director, Cancer Hospital and Research Institute, Gwalior for testing biological activities and Director, D.R.D.E., Gwalior for providing spectral data and elemental analysis and Principal, Govt. SMS Science College, Gwalior for providing research facilities.
References
- Buu-Hoi, Rene Royer, J.J. Jouin, J. Lecocq and D. Guettier, Bull. Soc. Chim., France, 128-36 (1947).
- Shichiro, Kakimoto and Kenichi Yamamoto; Pharm. Soc. Japan, 74, 997-9 (1954) ; C.A. Vol. 49, 11650 g (1955).
- L. Stoicescu – Crivetz and L. Mandasescu, Acad. rep. populare Romine, Studii, Cercetare, Chim. 4, 175-82 (1956); C.A. Vol. 51, 10421 h (1957).
- Agarwal S.K., Chandra R., Gupta R., Tutlani D.R.; J. Inst. Chem. (India), 59 (5) 225 (1987); C.A. Vol. 108, 164594 w (1988). Swiss 419, 139 (1967); C.A. Vol. 68: 29709 (1968).
- R. Cavier and R. Rips (Fac. Pharm, Paris), J. Med. Chem 8(5): 706-8 (1965).
- Martynovskii A.A., Samura B.A. and Co-workers, Med. Inst. Zaporzne, USSR, Khim. Farm. Zh. 24 (7), 31-2 (1990), C.A. 113: 164948 t (1990).
- Strokin, Yu. V., Karasovskii, I.A. and co-workers, (Bashk. Med. Inst., UFA, USSR), Khim. Farm. Zh, 24(7) 45-8 (1990), C.A. 114: 172 e (1991).
- Gardner, T.S.; Wenis, E.; and Lee, J.; J. Org. Chem., 26: 1514 (1961).
- Willy, R.H.; Slaymaker, S.; and Krans H.; J. Org. Chem. 22: 204 (1957).
- Omar, A.; Mohsen, M.E.; Farghaly, A.M.; Hazzai, A.A.B.; Eshba, N.H. (Fac. Pharm. Univ. Alexandria, Egypt). Pharmazie (1980); C.A., 95: 25382 a (1981).
- Bhat, A.K.; Bhamaria, R.P.; Patel, M.R.; Bellare, R.A.; and Deliwalia, C.V.; Indian J. Chem. 10: 694 (1972).
- Rollas,S.; Kucukguzel, S.G.; Molecules, 12: 1910-1939 (2007).
- Rollas S.; Gulerman, N.; Erdeniz, H.; Farmaco 57: 17 (2002).
- Kaymakcioglu K.B.; Oruc, E.E.; Unsalan, S.; Kandemirli,F.; Shvets, N.; Rollas, S.; Anatholy, D.; Eur. J. Med. Chem , 41: 1253-1261 (2006).
- Kalsi, R.; Shrimali, M.; Bhalla, T.N. ; Barthwal, J.P.; Indian J.Pharm. Sci. 41: 353-359 (2006).
- Ragavendran, J.; Sriram,D.; Patel,S.; Reddy,I.; Bharathwajan,N.; Stables, J.; yogeeswari, P.; Eur. J. Med. Chem. 42: 146-151 (2007).
- Salgin-Goksen, U.; Gokhan-Kelekci, N.; Goktas, O.; Koysal, Y.; Kilic, E.; Isik, S.; Aktay,G.; Ozalp, M.; Bioorg. Med. Chem. 15: 5738- 5751 (2007).
- Kucukguzel, S.G.; Mazi, A. ; Sahin, F.; Ozturk S.; Stables, J.P.; Eur. J. Med. Chem., 38: 1005- 1009 (2003).
- Loncle, C.; Brunel, J.; Vidal, N.; Dherbomez, M.; Letourneux, Y.; Eur. J. Med. Chem., 39: 1067-1071 (2004).
- Masunari, A.; Tavares , L.C.; Bioorg. Med. Chem., 15: 4229-4236 (2007).
- Sriram, D.; Yogeeswari, P.; Madhu, K.; Bioorg. Med. Chem. Lett. 15: 4502- 4505 (2005).
- Bijev, A.; Lett.Drug Des. Discov., 3: 506-512 (2006).
- Nayyar, A.; Jain, R.; Med. Chem., 12: 1873-1886 (2005).
- Scior,T.; Garces-Eisele, S.J.; Curr.Med. Chem., 13: 2205-2219 (2006).
- Janin, Y.; Bioorg. Med. Chem., 15: 2479-2513 (2007).
- Ulusoy, N.; Gursoy, A.; Otuk, G.; Kiraz, M. Farmaco, 56: 947-952 (2001).
- Linhong j, Jiang C, Baoan S, Zhuo C, Song Y, Qianzhu L, Deyu H and Ruiqing X, Bioorg Med Chem Lett., 16: 5036-5040 (2006).
- Caleta I, Grdisa M, Mrvos DS, Cetina M, Tralic KV, Pavelic K and Karminski G IL Farmaco, 59: 297-305 (2004).
- Geoffrey W, Tracey DB, Patrizia D, Angela S, Dong FS, Andrew DW and Malcolm FGS, Bioorg Med. chem. Lett., 10: 513-515 (2000).
- Terzioglu N., Gursoy A., Eur. J. Med. Chem., 38: 781-786 (2003)
- Singh V., Srivastava, V.K., Palit G., Shanker K.,Arzneim- Forsch. Drug. Res. 42: 993-996 (1992).
- Savini L., Chiasserini L., Travagli V., Pellerano C., Novellino E., Cosentino S., Pisano M.B., Eur. J. Med. Chem., 39: 113-122 (2004).
- Kalsi R., Shrimali M., Bhalla T.N., Barthwal J.P., Indian J.Pharm. Sci., 41: 353-359 (2006).
- Bernardino A., Gomes A., Charret K., Freitas A., Machado G., Canto- Cavalheiro M., Leon L., Amaral V., Eur. J.Med .Chem., 41: 80-87 (2006).
- Silva A.G., Zapata-Suto G., Kummerle A.E., Fraga C.A.M., Barreiro E .J., Sudo R.T., Bioorg Med. Chem. 1: ,3431-3437 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









