Synthesis and Characterization of 2-[2-Aryltelluroethyl] -3-Methyl Pyridines (Te,N) and 2-[2-(3-Methyl Pyridoethyltelluro) Ethyl]-3-Methyl Pyridine (N,Te,N)
K. K. Sharma1, Prarthana Srivastava2, Pragya3 and Kumud4
1Department of Chemistry, IMS, Ghaziabad, (India).
2Department of Chemistry, Krishna Institute of Engineering And Technoloy, Muradnagar (India).
3Department of Chemistry, Meerut College, Meerut (India). 4Adarsh Nagar, Modinagar, (India).
The potentially bidentate hard and soft acid containing (Te,N) compounds, 2-(2-aryltelluroethyl)-3-methyl pyridines (L) [C14H15NTe] and tridentate(N,Te,N) 2-[2-(3-methyl pyridoethyltelluro)ethyl]-3-methyl pyridine (L’) [C16H20 N2Te] have been synthesized in good yield ( > 75%) and characterized by physical, analytical and spectroscopic [1H, 13C{1H} and 125Te{1H} NMR and IR ] methods. The reaction of methyl iodide with L [C14H15NTe] results in the formation of the salt of the type ArTe (CH2) 2-2-(C5H4N)+. CH3I with affecting the tellurium moiety.
KEYWORDS:Ligands (Te, N) and (N, Te, N); IR spectra; NMR spectra
Download this article as:| Copy the following to cite this article: Sharma K. K, Srivastava P. Pragya, Kumud. Synthesis and Characterization of 2-[2-Aryltelluroethyl] -3-Methyl Pyridines (Te,N) and 2-[2-(3-Methyl Pyridoethyltelluro) Ethyl]-3-Methyl Pyridine (N,Te,N). Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Sharma K. K, Srivastava P. Pragya, Kumud. Synthesis and Characterization of 2-[2-Aryltelluroethyl] -3-Methyl Pyridines (Te,N) and 2-[2-(3-Methyl Pyridoethyltelluro) Ethyl]-3-Methyl Pyridine (N,Te,N). Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23493 |
Introduction
There has been a recent interest in the ligand chemistry of tellurium1,2 but despite this growth of activity the literature contains few reports of bi-or multidentate ligands containing tellurium. Incorporation of the ditelluride group into a cyclic system may be expressed to enhance the reactivity at the Te-Te bond as a result of strain involving both entropic and enthalpic effect3. Here we report synthesis and characterization of 2-(2-aryltelluroethyl)-3-methyl pyridines (L) [C14H15NTe], where Ar = Phenyl (La) [C14H15NTe], 4-methylphenyl (Lb) [C15H17NTe], 4-methoxyphenyl (Lc) [C15H17ONTe], 4-ethoxyphenyl (Ld) [C16H19ONTe], and 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine (L’) [C16H20 N2Te].
Material and Methods
Tellurium tetrachloride, aryl tellurium trichlorides, diarylditellurides, diphenyldiselenides, 2-(2-chloroethyl) pyridine are the main starting materials used in this work. The methods of conductance and magnetic moment measurement are given. The instruments and techniques used to record IR and NMR [1H, 13C {1H}]. Chemicals were obtained from BDH, Aldrich, Strem and Sigma are used without purification. Organic solvents (BDH, Merck and Glaxo) were used after purification and drying (whenever required) by standard methods4-5.
Experimental
2-(2-chloroethyl) pyridine and diarylditellurides (aryl= phenyl, 4-methylphenyl, 4-methoxyphenyl, 4-ethoxyphenyl) prepared by the literature methods6-7. The detailed procedure for the synthesis of L and L’ are given below.
Synthesis of 2-[2-aryltelluroethyl]-3-methyl pyridines (La-d)
To the hot ethanolic (25cm3) solution of appropriate ditelluride (3 mmol), solution of sodium boro hydride (1.0 gm, 10 cm3 of 10% NaOH), added slowly in nitrogen atmosphere, till colourless solution of sodium aryl telluride formed. To this 2-(2-chloroethyl)-3-methyl pyridine (6 mmol dissolved in 5 cm3 ethanol) was added drop wise with vigorous stirring and the resulting solution further refluxed for one hour. The ligand (La-Ld) thus formed were extracted into chloroform (250 cm3). After removing the excess of chloroform, liquid form of the compound separated out, which was purified by column chromatography, using silica column and chloroform: hexane (5: 95; 10: 90) as elutent.
The high boiling liquids L [C14H15NTe] and L’ [C16H20 N2Te] are pale yellow to red in colour, soluble in nonpolar and polar organic solvents. On exposure to air they change their colour from yellow to dark red. The various physical and spectroscopic properties of L and L’ are discussed below:
Conductance and molecular weight IR spectra measurements
The molar conductance (/\m) values of ligand L [C14H15NTe] and L’ [C16H20 N2Te] (Table 1)
Synthesis of 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine (l’)
In a round bottom (100 ml) flask Tellurium powder (2 gm, 16 mmol) and sodium borohydride (5 gm dissolved in 10 cm3 of 10% NaOH) were refluxed in water ( 50 cm3) until the colourless solution of Na2Te was obtained. To this 2-(2-chloroethyl)-3-methyl pyridine (4.9 gm, 32 mmol) in 5 cm3 of ethanol was added drop wise and the content further refluxed for two hours with vigorous stirring. The compound extracted into chloroform (250 cm3), after removal of excess of chloroform, red colour liquid was obtained, washed with water and dried over anhydrous calcium chloride under vacuum.
Results and Discussion
Synthesis of 2-(2-Aryltelluroethyl)-3-methyl pyridines (L) and 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine (L’) in schematic reaction shown as
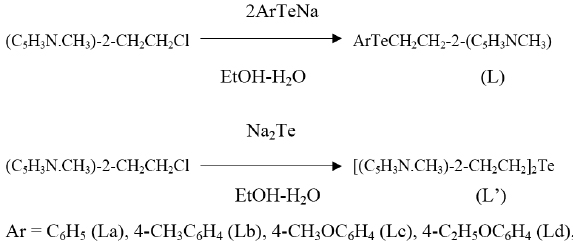
The high boiling liquids L [C14H15NTe] and L’ [C16H20 N2Te] are pale yellow to red in colour, soluble in nonpolar and polar organic solvents. On exposure to air they change their colour from yellow to dark red. The various physical and spectroscopic properties of L and L’ are discussed below:
determined in acetonitrile and nitro methane (1mM), reveals their nonelectrolytic behaviour. Molecular weights (Table 1) of L [C14H15NTe] and L’ [C16H20 N2Te] determined in chloroform have been found close to the molecular formulations arrived at by analytical data.
Conductance and molecular weight IR spectra measurements
The molar conductance (/\m) values of ligand L [C14H15NTe] and L’ [C16H20 N2Te] (Table 1)
The important IR bands along with their assignments and are presented in table 2. The assignments of the bands have been made on the basis of earlier reports8,10. The IR spectra of L [C14H15NTe] and L’ [C16H20 N2Te] show characteristic band at 410 cm-1 due to δ(C-N) vibration (out of plane ring deformation) (8). Two medium to low intensity υ [Te-C (aromatic)] bands appear between 240-260 and 280-295 in the IR spectra of L [C14H15NTe] concurring with earlier observations9. A band of medium to strong intensity at around 480 cm-1 in the IR spectra of L [C14H15NTe] and L’ [C16H20 N2Te] attributed to υ[Te-C(aliphatic)] vibration(9).
Table 1: Physical properties of ligands La-Ld and L’
| Compound | Color (Liquid) | Yield % | Bpt. (°C) | Solubility | Mol.wt. (Calcd.) | Te% (Calcd.)In CHCl3 | (/\M) In Ch3cn/C6h5no2 Ohm-1cm2mol-1 | |
| Good | Moderate | |||||||
| La [C14H15NTe] | Pale yellow | 85 | 172 | Hexane, | _ | 310 (324.6) | 38.20 (39.3) | 16.30/2.6 |
| Lb [C15H17NTe] | Yellow | 82 | 180 | Hexane,CHCl3CH3 CN | _ | 303 (338.6) | 37.50 (37.68) | 14.2/3.4 |
| Lc [C15H17ONTe] | Orange yellow | 85 | 178 | CHCl3,CH3CN | Hexane | 325 (354.6) | 35.93 (35.98) | 15.2/2.9 |
| Ld [C16H19ONTe] | Pale yellow | 90 | 184 | CHCl3,CH3CN | Hexane | 375 (368.6) | 34.0 (34.61) | 15,6/2.8 |
| L’ [C16H20 N2Te] | Red | 90 | 136 | Hexane,CHCl3CH3 CN | _ | 325 (367.6) | 33,60 (34.71) | 14.0/2.6 |
For 1:1 electrolyte /\m in acetonitrile = 100-160 and in nitrobenzene = 20-30 ohm-1cm2mol-1
La = C6H5TeCH2CH2–2-(C5H3NCH3) Ld = 4-C2H5OC6H4TeCH2CH2–2-(C5H3NCH3)
Lb = 4-CH3C6H4TeCH2CH2–2-(C5H3NCH3) L’ = [(C5H3N.CH3)-2-CH2CH2]2Te
Lc = 4-CH3OC6H4TeCH2CH2–2-(C5H3NCH3)
Table 2: Important ir bands (Cm-1) of L and L’
| Ligands | υ{Te-C(aliphatic)} | υ{Te-C(aromatic)} | δ (C-N) |
| La [C14H15NTe] | 480 | 262,292 | 410 |
| Lb [C15H17NTe] | 482 | 248,288 | 408 |
| Lc [C15H17ONTe] | 480 | 250,290 | 410 |
| Ld [C16H19ONTe] | 484 | 256,298 | 408 |
| L’ [C16H20 N2Te] | 497 | – | 410 |
Table 3. 1H and 125Te {1H} NMR data for 2-(2-chloroethyl) pyridine, La-Ld, and L’ in CdCl3 at25°c (δ, ppm)
| Compound | H6 (d) | Aryl and pyridyl protons (m) | H7(m) and H8 | H (R) 1H | 125Te{1H} |
| (C5H4N)-2-(CH2)2Cl | 8.45 | 7.20(H3,H5);7.58(H4) | 3.9t,3.2t | – | – |
| La [C14H15NTe] | 8.56 | 7.00-7.60 | 3.2 | – | – |
| Lb [C15H17NTe] | 8.51 | 6.95-7.70 | 3.2 | 2.38s | – |
| Lc [C15H17ONTe] | 8.5 | 6.70-7.70 | 3.2 | 2.75s | 481 |
| Ld [C16H19ONTe] | 8.55 | 6.70-7.70 | 3.2 | 3.97q, 1.40t | – |
| L’ [C16H20 N2Te] | 8.55 | 7.39(H3,H5);7.58(H4) | 3.3 | – | 167 |
La = 2-(2-Aryltelluroethyl)-3-methyl pyridine(s = singlet; d = doublet; t = triplet; q = quartet; m =multiplet)
Lb = 2-[2-(4-methylaryl) Telluroethyl]-3-methyl pyridine
Lc = 2-[2-(4-methoxyaryl) Telluroethyl]-3-methyl pyridine
Ld = 2-[2-(4-ethoxyaryl) Telluroethyl]-3-methyl pyridine
L’ = 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine
Table 4: 13C {1H} NMR chemical shifts for ligands La-Ld and L’ IN CDCl3 (δ, ppm)
|
Ligand |
C7 |
C8 |
Aryl and Pyridyl |
C(R) |
|
La* |
40 |
6.5 |
137.8(C2), 121.4(C ), 136.2(C ), 122.7(C ), 149.5(C ), |
– |
|
112.6(C9), 138.3(C10), 129.0(C11), 127.3(C12) |
||||
|
Lb |
40.1 |
3.2 |
134.0(C2), 117.7(C3), 135.0(C4), 119.0(C5), 149.5(C6), |
17.6 |
|
105.6(C9), 132.5(C10), 126.5(C11), 121.3(C12) |
||||
|
Lc |
40.3 |
6.8 |
140.0(C2), 121.3(C3), 136.3(C4), 122.7(C5), 149.5(C6), |
55.1 |
|
101.2(C9), 141.0(C10), 115.1(C11), 160.1(C12) |
||||
|
Ld |
40.2 |
6.8 |
140.0(C2), 121.4(C3), 136.4(C4), 122.2(C5), 149.5(C6), |
63.5,14.9 |
|
101.2(C9), 141.1(C10), 115.8(C11), 159.3(C12) |
||||
|
L’* |
40.3 |
2.2 |
136.5(C2), 120.2(C3), 135.6(C4), 122.3(C5), 148.2(C6) |
– |
* = Not Observed.
La = 2-(2-Aryltelluroethyl)-3-methyl pyridine [C14H15NTe]
Lb = 2-[2-(4-methylaryl) Telluroethyl]-3-methyl pyridine [C15H17NTe]
Lc = 2-[2-(4-methoxyaryl) Telluroethyl]-3-methyl pyridine [C15H17ONTe]
Ld = 2-[2-(4-ethoxyaryl) Telluroethyl]-3-methyl pyridine [C16H19ONTe]
L’ = 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine [C16H20 N2Te]
1H and 125Te{1H} NMR spectra
The 1H NMR spectra of Ligands 2-(2-aryltelluroethyl)-3-methyl pyridines (L) and 2-[2-(3-methyl pyridoethyltelluro) ethyl]-3-methyl pyridine (L’) were recorded in CDCl3 (11-13). The various chemical shifts along with their assignments are given in table 3.
The 1H NMR spectra of L [C14H15NTe] and L’ [C16H20 N2Te] show multiplet at ~δ,3.2for H7 and H8 rather than two triplets (centered at δ, 3.9 and 3.2 ppm respectively) as observed in the precursor, 2-(2-chloroethyl)-3-methyl pyridine. The up field shift (0.7ppm) of H8 in the ligand with respect to their precursor may be attributed to replacement of chlorine atom by tellurium, an atom of much lower electro negativity. This shielding of H8 protons result in their merger with H7 signals14-16.
The atomic ring protons in La [C14H15NTe] appear as multiplet and merged with the pyridine ring protons between δ 7.00-7.60 ppm. The aromatic ring protons of Lb-Ld [C15H17NTe], [C15H17ONTe], [C16H19ONTe], do not appear as two doublets as expected for a 1, 4-disubstituted benzene ring having substituents of different electro negativities(19) but merge with the pyridine ring protons and appear as a multiplet between δ 6.70-7.70 ppm.
In L [C14H15NTe] and L’ [C16H20 N2Te] the proton linked to the carbon ortho to nitrogen (H6) appears most downfield as compare to other ring protons and appears at δ, 8.5ppm as a doublet.
The nitrogen being electronegative in nature deshields the adjacent carbon most, and consequently the proton linked to it, is deshielded most17,18.
In L’, [C16H20 N2Te] H4 appear at δ, 7.25ppm, and H3 and H5 appear at ä, 7.08 ppm as multiplet as expected for ortho substituted alkyl pyridine20.
The 125Te {1H} NMR spectra of Lc [C15H17ONTe] and L’ [C16H20 N2Te] show a sharp singlet at ä, 481 and 167 ppm respectively which are in concordance with the values reported for asymmetric alkyl aryltellurides and symmetric dialkyltelluride(21-23).
13C {1H} NMR spectra
The assignment of the signals (Table 4) in the 13C {1H} NMR spectra of L [C14H15NTe] and L’ [C16H20 N2Te] recorded in CDCl3 have been made on the basis of literature reports on the related compounds and addivity principle. The C8 appears between δ, 2.2 and 6.8 ppm and C7 around δ, 40.0 ppm. The C8 have been found to be shielded because of the presence of a lone pair of electrons on the less electronegative tellurium atom in the ligand concurring with the earlier reports27 on such compounds.
This observation supports the shielding of CH2Te protons and consequently their merger with the C7 protons in 1H NMR spectra. The phenyl ring carbon linked to tellurium (i.e. C9) appears around δ, 100 ppm in the 13C {1H} NMR spectra of L [C14H15NTe] because it experiences greatest shielding among the phenyl carbons due to lone pair of electrons of the tellurium28. The carbons of the pyridine ring appear as expected for the ortho substituted alkyl pyridine. The carbon ortho to nitrogen (C6) appears at δ, 149.5 ppm, most downfield as compared to the other carbons of the ring. This is because of the greater electro negativity of the nitrogen which deshields C6 most; consequently it appears at most downfield29-31.
References
- H.J.Gysling. coord. chem. rev.42: 133 (1982).
- H.J.Gysling in F.J.Berry and W.R. Mcwhinnine (Eds.), 4th Inter. Conf. org.chem. Selenium and Tellurium Univ. Aston. Birmingham 32-82 (1983).
- M. Raves croft, R.M.G.Roberts and J.G.Tillett, J. chem.soc.Perkin Trans.II 1569 (1982).
- A.I.Vogel-practical organic chemistry, 3rd edn. (Longmans, London, 1975).
- J.A.Riddick and W.B.Bunger in organic solvents, vol.2, Techniques of chemistry, 3rd edn; Eds; Arnold Weissberger, (Wiley Interscience, New York) (1970).
- K.J. Irgolic and R.A. Zingaro-Reactions of organotellurium compounds in organometallic synthesis, vol. 2, Eds; E.Becker and M.Tsutsui (John Wiley and sons, Inc.1971).
- S.Okio and Y.Noike-J.pham.soc.Japan, 72: 490 (1952).
- V.K. Jain-Inorg.chem.Acta133 (1987)261, V.K. Jain, R.P. Patel, K.V. Muralidhara and R. Bohra. Polyhedron, 8: 2151(1989).
- A.K. Patra, M. Ray and R.N. Mukherjee-Inorg. chem. 39: 652 (2000).
- S. Chatel, Chauvin Anne-S, Ttuchagues, P. Jean, P. Leduc, E. Bill, C. Jean Chattard, D. Mansuy and J. Artaud- Inorg. chem. Acta 33: 19 (2002).
- H.C. Clark, V.K. Jain and G.S.Rao-J.organomet, chem., 279: 181 (1985).
- V.K. Jain and G.S.Rao, Inorg.chim.Acta, 127: 161 (1987).
- V.K. Jain, R.P. Patel, K.Venkatasubramanian-Polyhedron, 10: 851 (1991).
- C.E. Briant, C.J. Gardener, T.S. Andy Hor, N.D. Howells an D.M.P. Mingos- J.chem.soc. Dalton Trans. 2645 (1984).
- H.J. Gysling. Coord. chem. Rev., 42: 133 (1982), H.J. Gysling in S. Patai and Z. Rappaport(Eds.)- The chemistry of organo-selenium and Tellurium compounds, vol.1, Wiley, New York (1986).
- B.L. khandelwal, K. Kundu and S.K. gupta-Inorg.chim.Acta 154: 183 (1988).
- E.G. Hope, T. Kemmitt and W. Levason-Organometallics, 7: 78 (1988).
- T. Kemmitt and W. Levason- Organometallics, 8: 1303 (1989).
- P.K. Mascharak and D.S. Martin- Chem. soc. Rev., 29: 69 (2000).
- S.L. Jain, J.A. Crayto, D.T. Richen and J.A. Woollina-Inorg.chem.commun. 5: 853 (2002).
- S.K. Mandal and L. Que-Jr.Inorg.chem. 36: 5424 (1997).
- L.M. Barreaw, M.M. Makowaska, Grzyska and A.M. Arif- Inorg.chem. 39: 4390 (2000).
- T. Kiss, K. Petrohan, P. Buglyo, D. sanna, G. Micera, J.C. Pessoa and C. Maderia-Inorg.chem. 37: 6384 (1988).
- W.R. Mcwhinnie and M.C. Patel. J.chem.soc.Dalton Trans. 199 (1972).
- J.P. Renault, C. Verchere-Beaur and I. Morgenstern-Badarau. Inorg.chem. 39: 2666 (2000).
- H.M.K.K. Pathirana and W.R. Mchinnie-J.chem.soc. Dalton Trans. 2003 (1986).
- R.K. Chaddha and J.M. Miller-J.chem.soc. Dalton Trans. 117 (1982).
- W.J.Le Noble-J.Am.chem. soc.87: 2434 (1965).
- G. Petragnani and G. Schill-Chem.Ber.103: 2271 (1971).
- Sumit Bali, Ajai K. Singh, Pankaj Sharma, J.E. Drake, M.B. Hursthouse and M.E. Light, Inorganic Chemistry Communications, 6(11): 1378-1381 (2003).
- Garima Singh, Ajai K. Singh, Pankaj Sharma, John E. Drake, Michael B. Hursthouse, Mark E. Light, Journal of Organometallic Chemistry, 688(1-2): 20-26 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









