Spectral Studies on Cobalt (II) and Nickel (II) Complexes of Bis(1-Phenyl Tetrazoline)-5,5’-Disulphide
R. N. Pandey*, A. K. Nag, Prasashti Pande and Sanjay K. Singh
P.G. Center of Chemistry (MU), College of Commerce, Patna - 800 020 (India).
Bis(1-phenyl tetrazoline)-5,5’-disulphide forms stable complexes having general formula [CoA2 (ligand) 2] X and [Ni(H2O) 4 (ligand) 2] X (A = CH3COO – and H2O, X = ClO4 -, NO3 -, SO4 - -) at pH = 6. Cobalt (II) complexes are square planar having magnetic Bis (1-phenyl tetrazoline) –5,5’-disulphide forms stable complexes having general moment 2.74 – 2.76 BM. Sub-normal magnetic moment of octahedral Ni (II) complexes observed between 1.47 – 1.66 BM indicates square planar and octahedral equilibrium. The ligand field parameters n2 /n1 = 1.61, B’ = 764.9 cm-1, 10 Dq = 9943.7 cm-1 and B = 0.96 cm-1 also supports octahedral structure. IR spectra suggest bonding through one of the disulphide sulphur of ligand.
KEYWORDS:Heterocyclic organic disulphide; spectral properties Ni(II) and Co(II) complexes
Download this article as:| Copy the following to cite this article: Pandey R. N, Nag A. K, Pande P, Singh S. K. Spectral Studies on Cobalt (II) and Nickel (II) Complexes of Bis(1-Phenyl Tetrazoline)-5,5’-Disulphide. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Pandey R. N, Nag A. K, Pande P, Singh S. K. Spectral Studies on Cobalt (II) and Nickel (II) Complexes of Bis(1-Phenyl Tetrazoline)-5,5’-Disulphide. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23472 |
Introduction
Organic disulphides are very important class of organic compounds of great biological significance1. They have unique and interesting insights into structure and bonding and used as ligands by several workers2-6. The present study describes the complexes of Co(II) and Ni(II) with heterocyclic organic disulphide, bis(1-phenyl-tetrazoline)-5,5’-disulphide (Fig I)
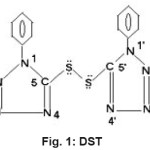 |
Figure 1: DST Click here to View figure |
Experimental
All chemicals were chemically pure grade. The ligand was prepared by the method described earlier7. Complexes were prepared using a general method. Alcoholic solution of metal salt was mixed ligand solution in benzene using appropriate molar ratios. The reaction mixture was then refluxed on water-bath and the volume of solution mixture reduced to ca.40 mL by evaporation. The complexes formed were digested on water bath for half an hour, centrifuged, washed with ice-cold ethanol and dried at 110oC in an electric oven.
Electronic absorption spectra of the complexes were recorded with the help of Cary, Model 17D Spectrophotometer n nujol mull. The magnetic measurements on all solid complexes were made at room temperature (300K) on the gouy balance. Calibration was done using Hg[Co(NCS)4] as standard. The conductivity of complexes were measured in DMF(10-3M) solution with the help of a systronic conductometer. Carbon, hydrogen and nitrogen analysis and IR spectra were recorded at CDRI, Lucknow. Analytical and physical data of complexes are given in TABLE 1.
Results and Discussion
Bis(1-phenyl tetrazoline)-5,5’-disulphide forms stable coloured solid having general formula [CoA2(DST)2]X and [Ni(H2O) 4 (DST) 2] X (A=CH3COO–&H2O, X=ClO4 –,NO3 –,SO4–) at PH=6. All complexes are insoluble in common organic solvents but fair solubility was attributed in DMF and solution of complexes in DMF (10-3M) supports their formulation8 (Table 1) . The magnetic moment of Co(II) complexes were found to be 2.74- 2.76 BM which corresponds square planar in structure9. A very intense absorption band at 27027 cm-1 in Co(II) complexes is assigned to charge transfer band either due to M→L or L→M. However, strong absorption bands at 16666 cm-1 and at 14285 – 14766 cm-1 indicate that these Co(II) complexes are square planar 10 and the bands are assigned due to 2A1g→ B1g and 2A1g→2B1g translations respectively.
The magnetic moment of Ni (II) complexes observed between 1.47-1.66 BM is intermediate between zero (square planar) and 2.83 BM (octahedral) probably due to square planar and octahedral equilibrium 11. Similar observations have been reported by Saha etal 12 and Benzer and Co-workers 13. If two water molecules associated with each Ni(II) ion revert to uncoordinated form to coordinated form and vice-versa then square planar and octahedral equilibrium is probable.
Electronic spectral bands and their assignment of Ni(II) complexes has been given in TABLE 2. All complexes display three major bands 14of weak intensity. This is the characteristic of octahedral Ni(II) complexes. The ν1/ν2 value lies between 1.61-1.64 supports the octahedral geometry to complexes and suggest a strong configuration interaction between the high spin T1g (P) and T2g(F) excited state. The value of â in the range 0.84-.96 puts the ligand (DST) towards the strong end of the nephelauxetic series. Schaffer et al 15 and Jorgensen16 have pointed out that ligands which coordinate through sulphur cause very pronounced nephelauxetic effects. Thus, coordination through sulphur may be suggested. It may be further noted that the v2 band is calculated to be equal to 12 Dq + 15 B’, which is equal to 23405 cm-1 in the case of [Ni(H2O)4(DST) 2]SO4. This is in good agreement with the observed band at 23239 cm-1. The other ligand field parameters ν2/ν1=1.61, B’=764.9 cm-1, 10 Dq = 9943.7 cm-1 and β = 0.96 of the complex also supports the octahedral structure 18.
Table 1: Analytical and physical data of complexes
|
Complexes/(colour) |
Magnetic Moment, BM |
Molar Conductance (ohm-1 cm2 mol-1) |
% Analysis: Found/(calcd) |
|||
|
C |
H |
N |
Metal |
|||
|
[Co(ac) 2 (DST) 2] |
2.75 |
9.8 |
43.5 |
3 |
25.4 |
7.1 |
|
(Blue) |
-43.3 |
-2.9 |
-25.3 |
-6.7 |
||
|
[Co(H2O) 2 (DST) 2](NO3) 2 |
2.74 |
145 |
36.5 |
2.6 |
26.9 |
6.4 |
|
(Blue) |
-36.3 |
-2.5 |
-27.1 |
-6.3 |
||
|
[Co(H2O) 2 (DST) 2]SO4 |
2.78 |
142 |
36.8 |
3 |
25 |
6.7 |
|
(Pink) |
-37.3 |
-2.7 |
-24.9 |
-6.6 |
||
|
[Co(H2O) 2 (DST) 2](ClO4) 2 |
2.73 |
144 |
33.6 |
2.2 |
22.8 |
6.1 |
|
(Black) |
-33.5 |
-2.4 |
-22.4 |
-5.9 |
||
|
[Ni(H2O) 4(DST) 2](ClO4) 2 |
1.47 |
139.3 |
32.2 |
3 |
21.4 |
5.8 |
|
(Greenish yellow) |
-32.3 |
-2.7 |
-21.5 |
-5.6 |
||
|
[Ni(H2O) 4(DST) 2](NO3) 2 |
1.56 |
176.6 |
35 |
3 |
26.2 |
6 |
|
(Green) |
-34.9 |
-2.9 |
-26.1 |
-6.1 |
||
|
[Ni(H2O) 4(DST) 2]SO4 |
1.67 |
141.3 |
35.8 |
3 |
24 |
6.5 |
|
(Light green) |
-35.9 |
-2.9 |
-23.9 |
-6.3 |
||
|
[NiCl2 (H2O) 2 (DST) 2] |
1.66 |
10.2 |
38.2 |
2.7 |
25.5 |
7 |
|
(Green) |
-38.4 |
-2.7 |
-25.6 |
-6.7 |
||
Infrared spectra of ligand (DST) and the complexes are similar from 3100cm-1 to 1600cm-1. The medium band at 1595 cm-1in the spectrum of the ligand assigned to νC = C + νC = N mode remains unchanged in position on complexation to metal ions indicating that C = C and C= N groups are not involved in coordination. The νC – S band of the ligands observed at 690 cm-1 split into two new bands on coordination.One band remains almost at the same position but other band is observed at 660-670 cm-1. This indicates that out of the C–S-S-C moiety of the ligand , one of the two S-atoms has coordinated with divalent metal ion while the other sulpher atom remains uncoordinated. This generates two νC-S bands, νC-S-M. The bonding through one of the disulphide sulphur atoms is also indicated by red shift of νS-S bond of ligand18 (500 cm-1) by 25 cm-1 on coordination.
The absorption associated with anions in these complexes are identified at 1085 cm-1 and 620 cm-1 for perchlorate 19, 1104 and 610 cm-1 for sulphate20 and 1360 and 810 cm-1 for nitrate21 and all these correspond to their uncoordinated nature. However, in the acetato complexes (COO) and (COO) stretching vibrations occurs at 1560 and 1440 cm-1respectively. The separation (∆ν – 120 cm-1) of these two bands is diagnostic of unidentate carboxylate coordination 22 of acetato group through its C-O – moiety.
Table 2: Electronic Spectral bands (in cm-1) and their Assignments
|
[Co(ac) 2 (DST) 2] |
27030 |
C T Band |
|
16670 |
2A1g →2B1g |
|
|
14285 |
2A1g→2Eg |
|
|
[Co(H2O) 2 (DST) 2](NO3) 2 |
26670 |
C T Band |
|
16665 |
2A1g→2B1g |
|
|
14705 |
2A1g→2Eg |
|
|
[Co(H2O) 2 (DST) 2]SO4 |
26680 |
C T Band |
|
16670 |
2A1g→2B1g |
|
|
14690 |
2A1g→2Eg |
|
|
[Co(H2O) 2 (DST) 2](ClO4) 2 |
26770 |
C T Band |
|
16670 |
2A1g→2B1g |
|
|
14660 |
2A1g→2Eg |
|
|
[Ni(H2O) 4(DST) 2](NO3) 2 |
31260 |
C T Band |
|
21276 |
3A2g(F)→3T1g(P) (ν3) |
|
|
{16666 14492 13335} |
3A2g(F)→3T1g(F) (ν2) |
|
|
9091 |
3A2g(F)→3T2g(F) (ν1) |
|
|
[Ni(H2O) 4(DST) 2]SO4 |
34480 |
C T Band |
|
23239 |
3A2g(F)→3T1g(P) (ν3) |
|
|
14706 |
3A2g(F)→3T1g(F) (ν2) |
|
|
9095 |
3A2g(F)→3T2g (ν1) |
|
|
[Ni(H2O) 4(DST) 2](ClO4)2 |
34480 |
C T Band |
|
25000 |
3A2g(F)→ 3T1g(F) (ν3) |
|
|
{16950 14758 13333} |
||
|
9091 |
3A2g(F)→!3T1g(F) (ν2) |
|
|
3A2g (F)→3T2g(F) (ν1) |
C T Band3A2g(F)→3T1g(P) (ν3) |
|
|
[NiCl2 (H2O) 2 (DST) 2] |
3127029415 |
|
|
{16666 142851 3333} |
3A2g(F)→3T1g(F) (ν2) |
|
|
9095 |
3A2g(F)→3T2g(F) (ν1) |
Far IR spectra of complexes contains new bands at 380 and 385 cm-1 assigned to νNi – S 23 and one cobalt-sulphur stretching mode 24 at 360 cm-1 indicates two bulky heterocyclic disulphide ligands are at trans position in square planar structure probably due to steric repulsion.
Thus, on the basis of aforesaid observations square planar configuration for Co(II) and octahedral structure for Ni(II) complexes is tentatively assigned.
References
- Pandey S.N., Sci. Reptr., 7: 661 (1970).
- Contreas J. G. and Cortes H., Inorg. Nucl. Chem. Lett,, 6: 225,639 (1970).
- Cotton F.A., .Frenz B.A, Hunter D.L. and Mester L.C., Inorg. Chim. Acta, 11: 111 (1974).
- Miller K., Inorg. Nucl. Chem. Lett, 14: 125 (1978).
- Mcquillan G.P. and Oxton I.A., Spectrochim. Acta, 35A: 865 (1979).
- Srivastava A.K., Agarwal R.K, . (Miss) Kapur Veena and Jain P.C., J. Indian chem.. Soc. 60: 498 (1983),.
- Pandey R.N., Sharma R.N., Sharma S.R. and Sahay A.N., Asian J. Chem. 4: 294 (1992).
- Geary W.J., Cord.Chem. Rev.7: 81 (1971).
- Cotton F.A. and Wilkinson G., “ Advanced Inorganic Chemistry” 4th. Edition, wiley, New York, 770,790 (1980).
- Nishida Y. and Kida S., Coord. Chem. Rev., 27: 275 (1979).
- Figgis B.N., ‘Introduction to Ligand Field theory’ John Wiley and Sons, Inc. New York, 319 (1966).
- Saha N. and Gayen N.C., J.Indian Chem. Soc., 60: 317 (1983).
- Benzer T.I., Dann L., Tamburro M.D. and Dudek E.P., Inorg. Chem., 10: 2204 (1971).
- Aderoju A. Osowole, Benjamin C. , Ejelonu and Saka A. Balogun, JUSPS, 20(3): 549 (2008).
- C.E.Schaffer, Abstracts, 140th National Meetings of the American Chemical Society 24N, Chicago, Illinois (1961).
- Jqrgensen C.K., Acta chem.. Scand 16: 2017 (1962).
- Lever A.B.P., Coord. Chem. Rev. 3: 119 (1968).
- Pandey R.N., Mrs. Kumari Rani and Kumar Narendra, Asian J. Chem., 7: 285 (1995).
- Bullitt J.G, Cotton F.A. and Marks J.J., Inorg. Chem., 11: 671 (1972).
- Hyms I.J., .Bailey R.T and Lippincoot E.R. Spectrochim Acta, 23A: 273 (1967).
- Addison C.C., Davis R. and Logan N., J. Chem. Soc., A, 3333 (1970).
- De A.K., De Deb, Nath Bhowmik K.R. and.Dutta R.N Purkayastha, J. Indian Chem. Soc., 86: 76 (2009).
- Watt J.W. and Mccrmick B.J., Spectrochim. Acta, 21: 753 (1965).
- D.C. Bradley, J. Chem. Soc., A, 1153 (1969).

This work is licensed under a Creative Commons Attribution 4.0 International License.









