Recent development of amino acids and peptides in metal ions detection: An overview
Mohd. Rashidi Abdull Manap, Nor Azah Yusof*, Siti Mariam Mohd. Nor and Faujan B. H. Ahmad
Department of Chemistry, Universiti Putra Malaysia 43400 UPM Serdang, (Malaysia)
Article Received on :
Article Accepted on :
Article Published : 02 Mar 2010
Little work has been reported on the development of solid state metal ion sensor based on the use of amino acids and peptides. This review covers literature on the use of amino acids and peptides (short peptide, oligopeptide and cyclic peptide) as a recognition molecule for metal detection system. Amino acids and peptides offer a high degree of selectivity, good limit of detection and high sensitivity towards detection of metal ion.
KEYWORDS:Amino acid; Peptides; Metal ions detection
Download this article as:| Copy the following to cite this article: Manap M. R. A, Yusof N. A, Nor S. M. M, Ahmad F. B. H. Recent development of amino acids and peptides in metal ions detection: An overview. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Manap M. R. A, Yusof N. A, Nor S. M. M, Ahmad F. B. H. Recent development of amino acids and peptides in metal ions detection: An overview. Available from: http://www.orientjchem.org/?p=11497 |
Introduction
Certain heavy metals can pose potential health risks when consumed by humans due to their high toxicity. Heavy metals such as Hg2+, Pb2+ and Cd2+ can be released into the environment through industrial use and erosion of natural deposits. Once it released to the environment, it can contaminate water, soil and air. These metals are very harmful to humans and have negative impact on the environment1.
Abundant references concerning the determination of metal with chemical sensors were available in literature. Table 1 showed some chemosensors used for metal ion detection. Some of the chemosensors have a low stability of a metal complex over time, high solubility only in organic solvent and also difficulty during response measurement.
Amino acids and peptides promise a bright chance for metal ion detection because it is usually soluble in aqueous medium and it can easily interact with metal. Peptides can be effective for metal ion sensor due to specificity and because they contain of potential donor atoms through the peptide backbone and also on the amino acid side chains. The donor’s atoms can be carbonyl oxygen or amide nitrogen and terminal amine. Polymeric character of peptide permits polydentate chelation which can provide strong binding and fast kinetic with ion. Selectivity and sensitivity were related with side chain of the amino acid in the peptide and can be optimized by further amino acid replacement during peptide synthetic strategy.
The aim of this review is to explore the use of amino acid, short peptide, oligopeptide and cyclic peptide for metal detection. Limit of detection and selectivity/interference of the sensors will be discussed. One letter abbreviation of amino acids were used in this review.
Amino acids
Amperometric method was used in order to trace Hg2+ in water7. The research focused on the usage of platinum electrode (high positive
potential) on the effect of the presence of mercury ions on the current due to oxidation of amino acid L-Tyrosine. The detection limit was 0.014 mM with relative standard deviation (RSD) 2.2%. The system used 20 µM L-Tyrosine, applied potential + 0.75V, 0.1 M phosphate buffer at pH 7. During interference studies, different metal have been used, among them are Zn2+, Ag+, Cu2+, Ni2+, Cd2+, Pb2+, Co2+, Fe2+ and Fe3+. Only Co2+ showed a significant interference at a concentration higher than that of Hg2+.
An optical approach (fluorescence study) was reported in development of a water soluble sensor L-Cysteine-capped ZnS Quantum Dots(QD) for detection of Cu2+ 8. The sensor developed was not sensitive towards Co2+ and Mn2+. QD have various advantages, it can overcome problem from organic dyes molecule, have a high photochemical stability and resistant to photo degradation. As the pH increase, the deprotonation of thiol group in the L-Cysteine occurred and resulting fluorescence intensity increased. As a result, the bond strength between Zn and L-Cysteine molecule become high. But at too high pH, fluorescence decrease due to the formation of Cu(OH)2. Detection limit of this probe was 7.1 × 10-6 M. The luminescence intensity of the L-Cysteine-capped ZnS QDs is minimally affected by Fe2+ and Ag+, but at very low ratio 1:100 (Cu2+ : Interference Ion).
Voltammetric study for detection of Cd2+ was carried out using mercury electrode in the presence of cysteine9. 0.1 M KClO4 has proven to be a good supporting electrolyte for the Cd2+ detection system. The system developed is able to detect 5.62 mg/ml of Cd2+. Heterocyclic thiophene and benzoxazole attached with alanine has been synthesized and characterized as a fluorescent probe10. The fluorescent probe developed did not show any change in the absorption and emission upon addition of Na+, Ca2+, Zn2+, Cu2+ and Ni2+. Deprotection of ester group at carboxylic has caused the probe to respond towards Cu2+, Ni2+ and Hg2+. This selective deprotection increased the coordination stability of metal complex. The S atom from the thiophene was believed to have complexation with Hg2+. The removal of protecting group on N terminal has caused the amine group become protonated and addition of Cu2+, Ni2+ and Hg2+ decreased the fluorescence intensity.
Metallothionein (MT), a family of cysteine-rich was used as a biosensor for Pd2+ 11. 0.05 M KCl was found as the best supporting electrolyte for determination of Pd2+. The sensitivity of the biosensor not only depends on the adsorptive transfer stripping (AdTS) but also on affinity of MT to Pd2+. The ability of the biosensor was tested on human urine and human blood sample with limit of detection of 0.8 µM Pd2+.
Short peptide
A fluorescent peptide consists of 9-carbonylantracene (AN) as fluorophore for the tripeptide Glycyl-Histidyl-Lysine (GHK) has been synthesized12. It was selective towards Cu2+. Little or no change in the fluorescence emission can be seen upon addition of 0.1 mM of Fe2+, Co2+, Ni2+ and Zn2+. In the presence of Cu2+ at a much lower concentration (10-6M), the fluorescence of GHK-AN was quenched down to 34% of its original intensity.
Hepel et al 13 investigated the immobilized film of tripeptide glutathione Glycyl-Seryl-Histidine (GSH) on a cysteamine-SAM (CA-SAM) formed on Au piezoelectrodes by adsorptive dissociation of a disulphide, cysteamine. By using electrochemical quartz cystal nanogravimetry (EQCN), voltammetric analyses of Hg2+ at Au/CA and Au/CA-GSH have been analyzed. For Au/CA-GSH piezosensor, a single mercury electrooxidation peak was observed. Meanwhile for bare Au and Au-CA piezosensors, multiple peaks were observed. This was a possibility of extensive interaction of mercury species with various groups of this multifunctional film. Mass to charge ratio plots indicate predominant ingress/ egress of Hg2+ to/from the film. Electronic structure in form of (CA)2Hg2+ revealed that two CA molecules adsorbed on Au in near-vertical and two nitrogen from CA attached to Hg2+. The functional groups (free sulfhydryl, amine, carboxylate) introduced to the film with tripeptide glutathione can be utilized in designing a sensor.
Table 1: Some of chemosensors for metal ion detection
| Chemosensors | Selectivity | Disadvantages |
| 8-hydroxyquinoline2 | Cu2+ | Unstable complex |
| Conjugated polymer3 | Mg2+ | Solubility in THF |
| SNS4 | Cr3+ | Solubility in THF |
| Calix[4]crown5 | Pb2+ | Solubility in CH3 CN |
| Metal ion templated resin6 | Pb2+ | Difficult measurement |
Tripeptide Glycyl-Glycyl-Histidine (GGH) modified electrode was reported based on its analytical performance14,15. Tripeptide electrode was modified by self-assembling mercaptopropionic acid (MPA) onto the gold electrode followed by covalent attachment of the tripeptide to the self assembled monolayer using carbodiimide coupling. The peptide modified electrodes were found to exhibit high sensitivity to Cu2+ in the range of 0 to 30 pM of Cu2+ and 5 nM of Cu2+ 15. In analytical performance aspect, the sensor could be regenerated for twenty times. The tripeptide electrode was used todetermine 0.12 ppm of Cu2+ in real sample. The interference of Ni2+ to this modified electrode was only minor.
Chow et al 15 also reported on the characterization of the tripeptide modified electrode. The characterization was focused on the transformation from bare Au surface to the MPA-GGH peptide. The sensor surface was modified via the formation of mercaptopropionic acid self-assembled monolayer (MPA SAM) followed by 1-ethyl-3-(3 dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) activation and attachment of the peptide. It was found that, the response time was short (less than 5 minutes) for this sensor. MPA-GGH modified electrode selective towards Cu2+. Pb2+ and Cr3+ were identified as significant interfering ions based on Plackett-Burman experimental design. The significant interfering could be related to the hard or soft atom properties of the ions. Figure1 showed the fabrication of MPA-GGH gold electrode and complexation with Cu2+.
High resolution differential surface plasmon resonance (SPR) was combined with highly selective cysteamine-modified peptide as molecular recognition element where it can specifically sense Cu2+ and Ni2+ ion16. Two immobilization methods were used which are 11-mercaptoundecanoic acid, (MUA)-NHS activation method and chemisorption through thiol bond for cysteamine. Combination of His6 with MUA-NHS activation monolayer was able to detect Ni2+ as low as 10.4 ppt, however chemisorption offered the highest sensitivity as low as 2.4 ppt Ni2+.
Meanwhile for GGH modified SPR sensor (chemisorption method) was able only to detect 0.1 ppb of Cu2+. MUA-NHS activation of GGH was able to detect until 2.0 ppt of Cu2+. Selectivity study showed that His6 is selective only towards Ni2+ but not for GGH. Meanwhile GGH is selective for both Ni2+ and Cu2+. GGH cysteamine modified electrode reported to have better dynamic range. It has been applied for determination of Cu2+ from tap water with detection limit of 0.34 ppm.
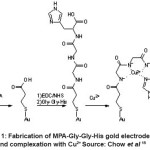 |
Figure 1: Fabrication of MPA-Gly-Gly-His gold electrode and complexation with Cu2+ Source: Chow et al 15 Click here to View figure |
Cyclic peptide
N, N’, N”-Trisubstituted-cyclo-triglycines were synthesized and exploited by Hioki et al17. They studied on potentiometric ion selectivity coefficient of the electrodes. Only two electrodes of these showed a great affinity towards Ca2+ over other cations. Molecular modeling showed one or two benzylic oxygens are outside cavity in some rotational isomers of amide linkage. Detection limit of the modified electrode was 1 ×10-5 M of Ca2+.
Incorporating different soft and hard atoms in the chelating ring promoting the stability and selectivity of the resulting complex. Copper poly(vinyl chloride) matrix membrance sensors based on cyclic tetrapeptide ionophores were prepared and characterized18. Two ionophores were used, Ionophore I is hydrophobic with less ring size and Ionophore II is more polar due to the presence of asymmetric substitution group, an ester, as shown in Fig 2. The sensors exhibit fast response, wide working pH range, high sensitivity, long-term stability and good selectivity. Different plasticizers affect the detection limit of the ionophores. The sensors have a detection limit of 0.05-0.13 µgml-1. The sensors have been applied for determining Cu2+ in ores and industrial wastewater.
Oligopeptide
Polyalanine peptide consists of twenty alanine residues for metal detection has been reported19. The oligopeptide can interact with monovalent (Li+, Na+, K+, Cs+ and R+) and dwivalent (Mg2+, Sr2+ and Ba2+) ion, but not with trivalent ion (In3+, Sc3+andY3+). In the unsolvated complex, the polyalanine peptide adopts a helical conformation that which stabilized by coordination of the metal ion to the C-terminus. Polyalanine peptide was helical in the Alan + M2+ complexes, but substantial disruption at the C-terminus where the metal ion bound. The interaction between the unsolvated peptide and metal ion increase as the charge ion increase. But not in the case of trivalent metals where it has high hydration energy and may not recovered by interaction with peptides, thus resulting no complex formation with peptide.
Conducting polymer was attractive because it can directly convert the binding event into an electrical signal. Aguilar et al 20 developed a sensor that was sensitive to Cu2+ and Ni2+ in ppt range by fabrication of polymer nanojunction on Si attached with peptide. GGH and His6 peptides were incorporated into the polymer nanojunctions.Nanojuntion is formed by bridging a pair ofnanolectrodes with electrodeposited peptide-modifed polyaniline (PANI). The system work based on the change in the nanojunction conductance when polymer conformational changes duringpeptide metal interaction. For poly (GGH-ANI) nanojuntion, decrease in current was seen upon addition of Cu2+. The detection limit was found to be 4 ppm of Cu2+. For the unmodified PANI, only little changes were observed during the process. Strong metal ion-peptide binding on the polymer nanojunction is due to the low dissociation constant16. This method has a high sensitivity and fast response. The nanojunctions are stable at room temperature at least six week and can be regenerate. Sensor was applied for detection of Cu2+ in drinking water sample.
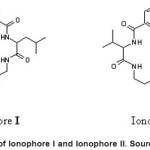 |
Figure 2: Structure of Ionophore I and Ionophore II. Source: Hassan et al 18 Click here to View figure |
White et al., 21 using nano magnetic material of γ-Fe2O3 and coated/immobilized with poly-L-Cysteine with 20 cysteine residues for chelation of As3+, Cu2+, Cd2+, Ni2+, Pb2+ and Zn2+. Poly-L-Cysteine-nano was inexpensive and showed strong highest binding towards Cu2+ and the lowest for Pb2+. Addition of poly-L-Cysteine increased the binding capacity towards these metals compared with no addition of peptide. The binding between poly-L-Cysteine –nano to metal increased with the increase of pH.
Another type of fluorescent probe based on the zinc finger consensus peptide (CP) that consists of twenty six linear amino acid arrangements was shown in Fig 3. This peptide showed a great response to Zn2+ 22. Modified CP with two fluorescent dyes, flourescein (F) as donor and lissamine (L) as acceptor group were used to “visualize” zinc binding. In the absence of Zn2+, the peptide was unfolded and the dyes were relatively far from each other.
The fluorescence emission spectrum contains two peaks (521 and 596 nm) that likewise correspond to fluorescein and lisamine. Upon addition of Zn2+, the fluorescence spectrum shift to a new wavelength, thus making the probe is ratioable.
Another example of peptidyl chemosensor was developed using combination fifteen residues of gylcine and aspartic acid in it sequence23. It consists of 2 flurophores, trytophan (Trp) as a donor and 5-(dimethylamino)naphthalene-1-sulfonyl (Dns) as acceptor for the probe. The probe had capability of Free Resonance Energy Transfer (FRET) between the Trp and Dns. Lack of a change in FRET efficiency indicates that the conformation of the peptide in this system was not changed as metal concentrations in the solution were altered. It was found that Cu2+ binding causes no change in FRET efficiency. This chemosensor was selective towards Cu2+ and detection limit was 32 µg L-1. Selectivity towards ions were tested with interfering ions such as Cd2+, Mn2+, Ni2+, Co2+, Zn2+ and Cu2+. Fluorescence intensity decrease only upon addition of Cu2+ and this bring significant towards selected metal compared to other metals.
Another fluorescent peptide probe developed based on ratiometric and selective towards Pb2+ 24. Two tetrapeptides were developed, Glutamyl-Cysteinyl-Glutamyl-Glutamic Acid (ECEE) and Glycyl-Glycyl-Glycyl-Glycyine (GGGG) Each probe consists of a fluorescent dye of dansyl group. Upon binding Dns-ECEE with Pb2+, the polarity of the environment surrounding changes and caused a concomitant shift in the fluorescence emission spectrum but not with Dns-GGGG. Ratiometric study was conducted by developing a calibration graph based on the ratio of fluorescence emission intensity before (557 nm) and after addition of metal to the system (510 nm). Shift in the emission spectrum only occurred in the system Dns-ECEE, but not in the Dns-GGGG. This probe behaves reversibility by addition of ethylenediaminetetraacetic acid (EDTA). Selectivity phenomena were observed during addition of Ca2+, Zn2+ or Cd2+. It was shown by the researchers that the probe was selective only towards Pb2+ even tough in the presence of either equimolar Zn2+ or equimolar Ca2+.
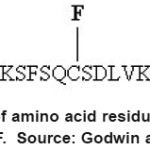 |
Figure 3: CP consists of amino acid residues and fluorescent dyes of L and F. Source: Godwin and Jeremy 22 Click here to View figure |
Bi et al., 25 reported the preparation and application of oligopeptide-modified single crystal silicon nanowire (SiNW) arrays as multichannel metal ion sensor. Surface of SiNW cluster was modified with different oligopeptides. Peptides were immobilized onto the SiNW clusters. Cysteinyl-Aspartyl-Arginyl-Valyl-Tyrosyl-Isoleucyl-Histidyl-Prolyl – Pheneylalanyl – Histidyl – Leucine (CDRVYIHPFHL) oligopeptide was reported to be sensitive towards Pb2+ meanwhile GGH was sensitive towards Cu2+. Selectivity towards Pb2+ could be explained by possibilities of Pb2+ to bind with Histidine residues and two adjacent carbonyl groups. High selectivity of GGH for Cu2+ can be attributed to the formation of three fused chelate rings and flat 4N coordination around Cu2+. By modifying each SiNW cluster with a different oligopeptide, it can be used as a multichannel metal ion sensor.
Joshi et al., 26 synthesized three florescent peptide probes for the detection of metal ion which is easily prepared through solid phase peptide synthesis (SPPS). The peptide consists of natural amino acid of histidine (H), cysteine (C), glutamic acid (E), proline (P) and glycine (G), Dansyl group acted as a flurophore for the probe. The probes were PG2 Dns-CPGHPGE-NH2, PG1 Dns-CGGHPGE-NH2 and GG2 Dns-CGGHGGE-NH2. The strategy was to develop prolyl-glycine (PG) sequence to stabilize a turn structure in the peptide and glycyl-glycine (GG) sequence was to adopt a random coil in the peptide structure. Results showed that the secondary structure played important role for selectivity and pre-organized secondary was not required for selective detection of Cu2+ but not for the detection of Zn2+. A GG2 probee was selective towards Cu2+ due to the two GG sequences in the peptide. Meanwhile for PG1, it was selective towards Zn2+ and Cu2+ because of one PG sequence in the peptide.
Dan-Glycyl-Glycyl-Histidyl-Glycine (Dns-GGHG) peptide was synthesized by Zheng et al.,27. The developed chemosensor was selective only towards Cu2+. Chemosensor was reported to interact with Cu2+ in the system at concentration as low as 1.0 µM. During the addition of Fe2+, Fe3+, Zn2+, Co2+ and Ni2+ to the chemosensor, the fluorescent intensity remained unchanged as like the chemosensor itself.
Conclusion
It seem that the development of amino acids and peptides as metal ion sensor promised a better detection system with abilities for low detection limit in the range of ppb and ppt. Peptide base sensors have been applied on real sample and the efficiency are comparable with other sensors. With high degree of selectivity and good sensitivity, peptides are indeed a promising ligand for multiple metal ions detection.
References
- Jarup L., British Medical Bulletin, 68: 167 (2003).
- Mei Y., Bentley P. A. and Wang W., Tetrahedron letters, 47: 2447 (2006).
- Ding A., Pei J., Yu W., Lai Y. and Huang W., Thin Solid Films, 417: 198 (2002).
- Sarkar M., Banthia S. and Samanta A., Tetrahedron letters, 47: 7575 (2006).
- Bok J. H., Kim H. J., Lee J. W., Kim S. K., Choi J. K., Tetrahedron letters, 47: 1237 (2006).
- Güney O., Yýlmaz Y. and Pekcan Ö., Sensors and Actuators B: Chemical, 85: 86 (2002).
- Majid S., Rhazi M. E., Amine A. and Brett C. M. A., Analytica Chimica Acta, 464: 123 (2002).
- Koneswaran M. and Narayanaswamy R., Sensors and Actuators B: Chemical, 139: 104 (2009).
- Tan W. T., Ooi L. L. and Lim E. B., Malaysian Journal of Chemistry, 3: 0013 (2001).
- Costa S. P., Oliveira E., Lodeiro C. and Raposo M. M., Sensors, 7: 2096 (2007).
- Adam V., Hanustiak P., Krizkova S., Beklova M., Zehnalek J., Electroanalysis, 19: 1909 (2007).
- Zheng Y., Huo Q., Kele P., Andreopoulos F. M., Pham S. M., Organic letters, 3: 3277 (2001).
- Hepel M., Dallas J. and Noble M. D., Journal of Electroanalytical Chemistry, 622: 173 (2008).
- Yang W., Chow E., Willett G. D., Hibbert D. B. and Gooding J. J., Analyst, 128: 712 (2003).
- Chow E., Wong E. L. S., Böcking T., Nguyen Q. T., Hibbert D. B., Sensors and Actuators B: Chemical, 111-112: 540 (2005).
- Forzani E. S., Zhang H., Chen W. and Tao N., Environmental Science & Technology, 39: 1257 (2005).
- Hioki H., Kinami H., Yoshida A., Kojima A., Kodama M., Tetrahedron letters, 45: 1091 (2004).
- Hassan S. S. M., Elnemma E. M. and Mohamed A. H. K., Talanta, 66: 1034 (2005).
- Kohtani M., Jarrold M. F., Wee S. and O’Hair R. A. J., The Journal of Physical Chemistry B, 108: 6093 (2004).
- Aguilar A. D., Forzani E. S., Li X., Tao N., Nagahara L. A., Appl. Phys. Lett., 87: (2005).
- White B. R., Stackhouse B. T. and Holcombe J. A., Journal of Hazardous Materials, 161: 848 (2009).
- Godwin H. A. and Berg J. M., Journal of the American Chemical Society, 118: 6514 (1996).
- White B. R. and Holcombe J. A., Talanta, 71: 2015 (2007).
- Deo S. and Godwin H. A., Journal of the American Chemical Society, 122: 174 (2000).
- Bi X., Agarwal A. and Yang K., Biosensors and Bioelectronics, 24: 3248 (2009).
- Joshi B. P. and Lee K., Bioorganic & Medicinal Chemistry, 16: 8501 (2008).
- Zheng Y., Gattás-Asfura K. M., Konka V. and Leblanc R. M., Chemical Communications, 2350 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









