GC-MS Study and Isolation of A Sesquiterpene Lactone from Artemisia Pallens
A. D. Ruikar 1, M. M. Kulkarni1, U. D. Phalgune 2, V. G. Puranik
1Dr. T. R. Ingle Research Laboratory, Department of Chemistry, S. P. College, Pune - 411 030 (India)
2Central NMR Facility and cCentre for Materials Characterization, National Chemical Laboratory, Pune - 411 008 (India).
Artemisia pallens wall is a potant medicinal plant used in Ayurvedic system of medicines since ancient times. Taking into consideration the medicinal importance of the plant, a fraction of acetone extract was analyzed using GC-MS and the structures were confirmed by genesis. The major constitutents were alpha santonin, diisobutyl phthalate, tetradecane etc. The presence of alpha santonin and its isolation has been shown for the first time. The structure of the isolated compound has been elucidated by spectral analysis (MS, 1H-NMR, 13C-NMR, DEPT etc.). It is further confirmed by single crystal X-Ray crystallography.
KEYWORDS:GC-MS; sesquiterpene lactone; Artemisia pallens
Download this article as:| Copy the following to cite this article: Ruikar A. D, Kulkarni M. M, Phalgune U. D, Puranik V. G. GC-MS Study and Isolation of A Sesquiterpene Lactone from Artemisia Pallens. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Ruikar A. D, Kulkarni M. M, Phalgune U. D, Puranik V. G. GC-MS Study and Isolation of A Sesquiterpene Lactone from Artemisia Pallens. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23508 |
Introduction
Ayurveda is a 5000 year-old system of natural healing that has its origins in the Vedic culture of India. Medicinal plants and herbs contain substances known to modern and ancient civilizations for their healing properties.
Higher plants, as sources of medicinal compounds, have continued to play a dominant role in the maintenance of human health since ancient times1. Over 50% of all modern clinical drugs are of natural product origin2 and natural products play an important role in drug development programs in the pharmaceutical industries3.
A. Pallens is a small and aromatic herbaceous plant which is native to the southern part of India, especially to the states of Karnataka, Tamil Nadu, Andhra Pradesh and in Maharashtra. In the regional languages of the south, it is known by several names as “davanam” in Tamil, “davanamu” in Telugu and “davana” in Kannada. Its leaves and flowers are highly valued in the making of floral decorations and oils. Leaves are very small, bluish green with yellow flowers and inconspicuous. It is utilized in traditional Ayurvedic medicinal formulations. Oral administration of the methanol extract of the aerial parts of Artemisia pallens Wall (Used in Indian folk medicine for the treatment of diabetes mellitus) led to significant blood glucose lowering effect in glucose-fed hyperglycemic and alloxan-induced diabetic rats4. Essential oil of davana is useful as antiseptic and disinfectant5. The present work is carried out in order to evaluate phytochemicals of therapeutic potential.
Material and Methods
Plant Material
The plant material was collected from Jejuri, Maharashtra state, India. It was authenticated at Botanical Survey of India, Pune. Its authentication number is BSI/WC/Tech/2008/1059.
Extraction
Air shade dried and powdered plant material (100 g) was extracted with acetone by stirring for 18 hours at room temperature. Solvent was recovered under reduced pressure to obtain crude extract. The extract produced was 7.27 % The crude extract (7g) was broad fractioned on silica gel (60-120, 10 g) by n-hexane , non-polar solvent, with increasing percentage of acetone.Thus seven broad fractions( A – G ) were collected. The details of it are summarized in Table 1.
GC-MS of fraction (B) was carried out. Fraction (B) (650 mg) was adsorbed on 2 g silica and column was eluted through hexane: ethyl acetate with increasing polarity of ethyl acetate. Details of it are given in Table 2. The compound was further purified by repeated crystallization using mixed solvent system.
GC-MS analysis
Gas chromatography analysis was performed by Agilent 6890N with FID using HP-5 capillary column. GC-MS analysis was performed using a Shimadzu QP 5050A mass spectrometer coupled with a Shimadzu 17A gas chromatograph fitted with a split-splitless injector and a DB-5 fused silica capillary column (30m × 0.25 mm i. d., 0.25 µm film thickness). Helium was used as a carrier gas at a flow rate of 1.0 ml/min. The injection port was maintained at 250 oC, and the split ratio was 40:1 . Oven temperature programming was done from 50 to 280 oC, at 10 oC/min, and it was kept at 280 oC for 5 min. Interface temperature was was kept at 250 oC. Ionization mode was electron Impact ionization and the scanning range was from 40 amu to 400 amu. Mass spectra were obtained at 0.5 sec. Interval. The spectra of the compounds were matched with NIST and Wiley library. Their structures were defined by the % similarity values.
Results and Discussion
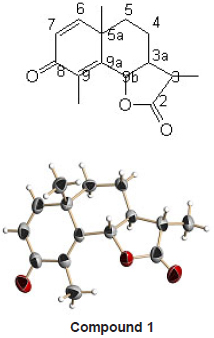
GC-MS indicates presence of different chemical constituents in it. The compounds identified are listed in Table 3. The major constituents were alpha santonin, tetradecane, hexadecane, diisobutyl phthalate, pentatriacontane etc. Out of these alpha santonin was isolated as a white crystalline solid. It showed sharp melting nature at 172 0C. LC-MS of the compound exhibited a molecular ion peak at m/ z 247 which suggested the molecular formula C15H18O3. The IR spectrum showed the absorption bands at 1785 cm-1 (lactone carbonyl), 1658 cm-1 (disubstituted alkene) and 1629 cm-1 (carbonyl group in conjugation).
Table 1: Broad Fractionation of Acetone Extract
| Fr. NO. | Eluent | Total volume collected (ml) | Weight (g) | Approximate composition |
| A | Hexane (100%) | 5 × 100 | 2.086 | Mixture of unidentified compounds |
| B | Hexane:Acetone (95:5) | 6 × 100 | 0.678 | Mixture of unidentified compounds + |
| compound 1 | ||||
| C | Hexane : Acetone (90:10) | 5 × 100 | 0.415 | Mixture of unidentified compounds |
| D | Hexane : Acetone (80:20) | 6 × 100 | 0.725 | Mixture of unidentified compounds |
| E | Hexane : Acetone (70:30) | 6 × 100 | 0.988 | Mixture of unidentified compounds |
| F | Hexane : Acetone (60:40) | 5 × 100 | 0.519 | Mixture of unidentified compounds |
| G | Hexane : Acetone (50:50) | 5 × 100 | 0.227 | Mixture of unidentified compounds |
Methyl protons at C-3 showed a doublet at 1.19 δ. Methyl protons of 5a at tetra substituted carbon showed a singlet at 1.27 δ. Methyl protons at C-9 (sp2 hybridized carbon) showed a singlet at 2.05 d. A doublet for lactone proton appeared at 4.77 δ. Doublets of olefinic protons on C-6 and C-7 are noticed at 6.67 and 6.18 δ respectively. A multiplet at 2.46 δ is observed for C-3 proton.
The 13C NMR reveled presence of total 15 carbon atoms. After recording DEPT spectrum it was clear that the molecule showed three quartets, one triplet, six doublets and five singlets. The structure of the compound was established on the basis of 1H and 13C NMR as [3S – (3, 3a , 5a, 9b )] – 3a, 5, 5a, 9b – tetrahydro- 3, 5a, 9 – trimethyl naphtha [1, 2 – b] furan – 2, 8 (3H, 4H) – dione.
Crystal Data for the Compound 1
Empirical formula C15 H18 O3
Formula weight 246.29
Temperature 273(2) K
Wavelength 0.71073 Å
Crystal system Orthorhombic
Table 2: Rechromatography of Fraction (B)
| Fr. NO. | Eluent | Total volume collected (ml) | Weight (g) | Approximate composition |
| B-1 | Hexane (100%) | 3 × 100 | 12 | Mixture of unidentified compounds |
| B-2 | Hexane : EthylAcetate (90:10) | 7 × 100 | 96 | Mixture of unidentified compounds |
| B-3 | Hexane : EthylAcetate (80:20) | 8 × 100 | 95 | Mixture of unidentified compounds |
| B-4 | Hexane : EthylAcetate (80:20) | 6 × 100 | 128 | Mixture of unidentified compounds + compound 1 |
| B-5 | Hexane : EthylAcetate (70:30) | 6 × 100 | 150 | Mixture of unidentified compounds |
| B-6 | Hexane : EthylAcetate (60:40) | 6 × 100 | 58 | Mixture of unidentified compounds |
Table 3: GC-MS Analysis Data
| Sr. No. | Retention Time ( minutes ) | Name of Compound | % Similarity | Molecular ion peak ( amu ) | Base peak ( amu ) |
| 1. | 8.9’ | Tetradecane | 96 % | 198 | 57 |
| 2. | 10.4’ | Hexadecane | 98 % | 226 | 57 |
| 3. | 11.8’ | Octadecane | 97 % | 254 | 57 |
| 4. | 12.4’ | Di-isobutyl phthalate | 94 % | 278 | 149 |
| 5. | 13.0’ | Pentatriacontane | 89 % | 492 | 57 |
| 6. | 15.0’ | Alpha santonin | 94 % | 246 | 173 |
Space group : P212121
Unit cell dimensions
a = 6.9962(5) Å α= 90°.
b = 10.7176(8) Å β= 90°.
c = 34.572(3) Å γ = 90°.
Volume : 2592.3(3) Å3
Density (calculated) : 1.262 Mg/m3
Crystal size : 0.33 x 0.10 x 0.06 mm3
Completeness to : θ 25.00° 99.9 %
Final R indices : R1 = 0.0438, wR2 = 0.0915
[I>2sigma(I)]
13C NMR Spectral data of the compound (CDCl3 at 100 MHz)
| Carbons | Chemical shift in ppm-d |
| C – 2 | 177.65 (s) |
| C – 3 | 40.87 (d) |
| C – 3a | 53.53 (d) |
| C – 4 | 23.06 (t) |
| C – 5 | 37.83 (d) |
| C – 5a | 41.38 (s) |
| C – 6 | 155.13 (d) |
| C – 7 | 125.86 (d) |
| C – 8 | 186.34 (s) |
| C – 9 | 128.68 (s) |
| C – 9a | 151.30 (s) |
| C – 9b | 81.40 (d) |
| C3 – CH3 | 12.51 (q) |
| C9 – CH3 | 10.93 (q) |
| C5a – CH3 | 25.14 (q) |
¹H NMR Spectral data of the compound (CDCl3 at 400 MHz)
| Protons | Chemical shift in ppm- δ |
| C3-CH3 | 1.19 (d, 6.8 Hz, 3 H) |
| C9-CH3 | 2.05 ( s, 3 H) |
| C5a-CH3 | 1.27 ( s, 3 H) |
| H-9b | 4.77 |
| H-6 | 6.67 (d, 10 Hz, 1 H) |
| H-7 | 6.18 (d, 10 Hz, 1 H) |
| H-3 | 2.46 (m, 1 H) |
Acknowledgements
Authors are thankful to the Principal and the Head, Department of chemistry, S.P. College, Pune, India for providing the necessary laboratory facilities for the work. Authors are also thankful to The Director, National Chemical Laboratory, Pune, India.
References
- Farombi EO. African J Biotech, 2: 662 (2003).
- Stuffness M, Douros J. J Nat Prod, 45: 1 (1982)
- Baker JT, Borris RP, Carte B et al. J Nat Prod, 58: 1325 (1995 )
- Subramoniam, A., Pushpangadan, P., Rajasekharan, S., Evans, D.A., Latha, P.G., Valsaraj, R. Journal of Ethnopharmacology, 50: 13 (1996).
- http://www.organicfacts.net/health-benefits/ essential-oils/health-benefits-of-davanaessential- oil.html.

This work is licensed under a Creative Commons Attribution 4.0 International License.









