Electrocatlytic oxidation of ascorbic acid mediated by ZNO microcrystalline modified glassy carbon electrode
WeeTee TAN1, Mohammed Zidan1, Zulkarnain Zainal1, Abdul Halim Abd
1Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM, Serdang, Selangor D.E., (Malaysia).
2School of Science, Monash University Sunway Campus, 46150 Bandar Sunway, Petaling Jaya, (Malaysia).
Article Received on :
Article Accepted on :
Article Published : 02 Mar 2010
Modification of a glassy carbon (GC) electrode surface by adhered microparticles of zinc oxide (ZnO) using electrochemical performance of microparticles of ZnO/GC electrode shows excellent electrocatalytic activity towards the oxidation of ascorbic acid in 0.1 M KH2PO4 electrolyte solution by cyclic voltammetry (CV). This paper seeks to critically examine the modification of GC electrode with Zinc oxide microparticles and the effect on oxidation of ascorbic acid using cyclic voltammetry technique. ZnO/GC electrode exhibited obvious enhancing and electrocatalyzing effect as it causes the oxidation current of ascorbic acid to increase by 1.5 times as compared to bare GC electrode. The sensitivity under conditions of cyclic voltammetry is significantly dependent on pH and ZnO dosage. The variation of scan rate study shows that the system undergoes diffusion-controlled process. Diffusion coefficient and rate constant of ascorbic acid were determined using hydrodynamic method (rotation disk electrode) with values of 5.4 x 10-6 cm2s-1 and 2.5x 10-3cms-1 respectively for unmodified electrode, while the values of diffusion coefficient and rate constant of ascorbic acid using ZnO/GC electrode were 5.7 x 10-6 cm2 s-1 and 2.1x10-3cms-1 respectively.
KEYWORDS:Zinc oxide Microcrystalline; Modified GC electrode; Ascorbic acid; Cyclic voltammetry
Download this article as:| Copy the following to cite this article: TAN W. T, Zidan M, Zainal Z, Abd A. H. Electrocatlytic oxidation of ascorbic acid mediated by ZNO microcrystalline modified glassy carbon electrode. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: TAN W. T, Zidan M, Zainal Z, Abd A. H. Electrocatlytic oxidation of ascorbic acid mediated by ZNO microcrystalline modified glassy carbon electrode. Available from: http://www.orientjchem.org/?p=11505 |
Introduction
ZnO is material of interest in modified electrode like other transition metal to show particular electrochemical and electrocatalytic behavior via electrooxidation of some substrates1. Modification of electrode surface with electroactive materials is an important area of research in electrochemistry for one decade2-4. Zinc oxide has been promising applications in catalytic, electrical, optoelectronic, photochemical fields and sensors5-7. In recent years, metallic nanoparticles have attracted much attention in electroanalysis because of their unusual physical and chemical properties including electrodeposition attachment of metal oxide particles and nanoparticles such as as Au8, Pt9,10, Ag11, Cu12, Ni13 and ZnO2 14, MnO2 15, SnO2 16, TiO2 in oxidation of some organic and biological substances17. The electrode surface can be modified with metal hexacyanoferrate by different ways such as electrodeposition, adsorption, mechanically attaching using gold electrode18-23. Also the metal hexacyanoferrate modified electrode have been widely used in electrochemistry to catalyze the oxidation of dopamine24, glutathione25-26, cysteine27. The analysis of ascorbic acid is important because it involves wide area of usage such as in drugs, and as chelating agents for heavy metals in body. Ascorbic acid is essential in human body due to its importance in antioxidant property. Ascorbic acid is a water-soluble type of organic acid, which is also known widely as vitamin C. Many studies have been reported on the catalytic oxidation of ascorbic acid28-34. In this paper, we are reporting the modification of GC electrode with zinc oxide microparticles using various voltammetric techniques. Finally, the electrocatalytic behavior of ZnO/GC electrode was investigated towards oxidation of ascorbic acid in 0.1 M KH2P04 electrolyte solution.
Experimental
Instrumentation and electroanalytical analysis methods
Electrochemical workstations of Bioanalytical System Inc. USA: Model BAS 50W with potentiostat driven by electroanalytical measuring software were connected to computer to perform cyclic voltammetry (CV), chronoamperometry (CC) and chronoamperometry (CA). Ag/AgCl (3M NaCl) and platinum wire were used as a reference and counter electrodes respectively. The working electrode used in this study was 3 mm diameter glassy carbon (GC). Unless otherwise stated, the voltammetric experiments were carried out at 25 ± 2oC using 0.1 M KH2P04 as supporting electrolyte. Solution was degassed with nitrogen gas for ten minutes prior to recording the voltammogram. Hydrodynamic voltammetry using rotating (carbon) disk electrode (RDE-1) and model: BAS-100A voltammetric workstation was also employed in this study.
Reagents
Zinc oxide was obtained from A Johnson Mattney Company, with 99.9% purity. All solutions were prepared using deionized water from reverse osmosis (RO) water model Elken (BIO PURE). Unless otherwise specified, the supporting electrolyte was 0.1 M KH2P04 in aqueous media at room temperature.
Preparation of zinc oxide modified glassy carbon electrode
The solid compound Zinc oxide (ZnO) was transferred to the surface of glassy carbon (GC) electrode as follows: Sample amounts of 1-3 mg of microcrystalline ZnO were placed on a coarse grade filter paper. The glassy carbon electrode was pressed onto the substance and rubbed over the material, causing some compound to adhere to the electrode surface. The clean glassy carbon surface could be renewed after the measurement by polishing with 0.5 µm alumina slurry, followed by ultrasonic cleaning for about 2-3 minutes, rinsing with distilled water.
Figure 1(a) shows the cyclic voltammograms of 0.5 mM ascorbic acid in 0.1 M KH2P04 supporting electrolyte at pH 4.5 using bare GC electrode. Modified GC electrode with ZnO microparticles shows the effect on the catalytic oxidation of ascorbic acid at the ZnO/GC electrode as shown in Figure 1(b) as it causes the oxidation current of ascorbic acid to increase by 1.5 times as compared to bare GC electrode. The oxidation of ascorbic acid is irreversible during cyclic voltammetry as there is an absence of electroactivity during the reverse scan.
Effect of pH
The pH was varied from pH 2.5 to 8 to determine its effect on the catalytic oxidation of 0.5 mM ascorbic acid at the ZnO/GC modified electrode. Figure 2 shows that the oxidation current of 0.5 mM ascorbic acid increases with an increase in pH between 2.7 to 4.5 with a maximum current response at pH4.5. However, current decreased gradually from pH 5 onward until pH 6.0
Effect of temperature
In general current markedly increased with an increase in temperature. Figure 3 shows the Arrhenium plot of log iox versus reciprocal of temperature using ZnO/GC electrode is obeyed for the oxidation of ascorbic acid over the temperature range of 30 to 80 0C. This indicates that the rate of the oxidation process of ascorbic acid species
increases as the temperature increases as expected. The temperature dependence may be associated with a change in conductivity and diffusivity of the solid because temperature has a significant influence on the energy formation of a compound. The effect of temperature on conductivity or diffusivity is given by equations (1) and (2).
s = s0 exp (-∆H/RT) …(1)
or
D = D0 exp (-∆H/RT) …(2)
where,
s / D = conductivity / diffusivity
s 0 / D0 = standard conductivity or initial diffusivity of the material
∆H = formation energy
So, conductivity or diffusivity would be predicted to change exponentially with temperature.
Table 1: Effect of amount ZnO attached onto a GC electrode on the oxidation of 0.5mM ascorbic acid in 0.1 M KH2PO4 (pH 4.5) electrolyte
| Amount of [ZnO]/mg | Ipa/(AA) | Enhancement further |
| 0 | 3.01 | – |
| 0.05mg | 3.65 | 1.2 |
| 0.1mg | 3.98 | 1.3 |
| 0.2mg | 5.43 | 1.8 |
| 0.3mg | 5.9 | 1.9 |
| 0.5mg | 6.2 | 2.0 |
Effect of varying amount of ZnO
The effect of varying the amount of microparticles ZnO attached on the 3 mm diameter glass carbon electrode surface ranging from 0.05mg to 0.5mg was studied. As shown in Figure 4, the results show that the use of increase amount of ZnO was associated with an increase in oxidation peak current of ascorbic acid and that of Zn peak at -0.1V as expected. However, 0.2mg was employed in this study because the use of a higher dosage would lead to a higher error due to the small electrode surface area.
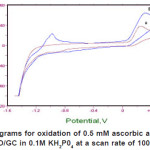 |
Figure 1: Cyclic voltammograms for oxidation of 0.5 mM ascorbic acid obtained at (a) bare GC electrode and (b) ZnO/GC in 0.1M KH2P04 at a scan rate of 100mV/s at 25°C and pH 4.5 Click here to View figure |
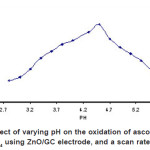 |
Figure 2: Effect of varying pH on the oxidation of ascorbic acid in 0.1 M KH2P04 using ZnO/GC electrode, and a scan rate of 100 mV/s Click here to View figure |
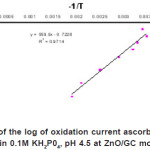 |
Figure 3: Dependence of the log of oxidation current ascorbic acid on reciprocal of temperature in 0.1M KH2P04, pH 4.5 at ZnO/GC modified electrode Click here to View figure |
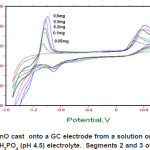 |
Figure 4: Effect of dosage ZnO cast onto a GC electrode from a solution on the oxidation of 0.5 mM ascorbic acid in 0.1 M KH2PO4 (pH 4.5) electrolyte. Segments 2 and 3 of experiments are shown Click here to View figure |
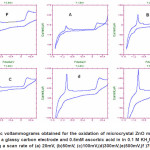 |
Figure 5.Cyclic voltammograms obtained for the oxidation of microcrystal ZnO mechanically attached to a glassy carbon electrode and 0.5mM ascorbic acid in in 0.1 M KH2P04 at pH4.5 using a scan rate of (a) 20mV, (b)50mV, (c)100mV,(d)300mV,(e)500mV,(f )700mV Click here to View figure |
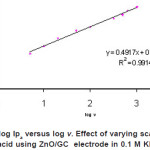 |
Figure 6: Plot of log Ipa versus log v. Effect of varying scan rates of 0.5 mM ascorbic acid using ZnO/GC electrode in 0.1 M KH2P04 at pH4.5 Click here to View figure |
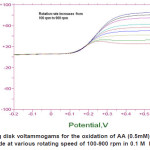 |
Figure 7: Rotating disk voltammograms for the oxidation of AA (0.5mM) using ZnO/GC modified electrode at various rotating speed of 100-900 rpm in 0.1 M KH2PO4 at pH 4.5 Click here to View figure |
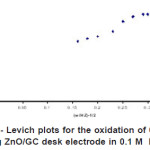 |
Figure 8: Kentucky- Levich plots for the oxidation of 0.5 mM ascorbic acid at rotating ZnO/GC desk electrode in 0.1 M KH2PO4 at pH 4 Click here to View figure |
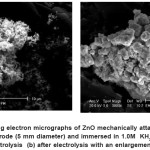 |
Figure 9: Scanning electron micrographs of ZnO mechanically attached to a basal graphite electrode (5 mm diameter) and immersed in 1.0M KH2PO4 electrolyte (a) before electrolysis (b) after electrolysis with an enlargement of 3000 times Click here to View figure |
Effect of Varying Scan Rate
The effect of varying scan rates (v) on the cyclic voltammograms of 0.5 mM ascorbic acid using modified ZnO/GC as working electrode in 0.1 M KH2P04 supporting electrolyte was studied over 20– 700 mV/s. The oxidation peak current of zinc oxide increase with scan rate and also the oxidation current of ascorbic acid was observed to increase with scan rate due to heterogeneous kinetics.
Figure 5.Cyclic voltammograms obtained for the oxidation of microcrystal ZnO mechanically attached to a glassy carbon electrode and 0.5mM ascorbic acid in in 0.1 M KH2P04 at pH4.5 using a scan rate of (a) 20mV, (b)50mV, (c)100mV, (d) 300mV, (e) 500mV, (f )700mV.
The log plot of peak currents at various scan rate are linearly related. Based on a plot of log (peak current) versus log (scan rate, v) for oxidation current of the first cycle, a straight line was obtained fulfilling the equation y = 0.49 x – 0.61 with R2=0.991. A slope of 0.49, which is comparable with theoretical slope of 0.5 for diffusion controlled process, was obtained.
Hydrodynamic Voltammetry Using Rotating Disk electrode (RDE)
The RDE technique was used to determine the diffusion coefficient and rate constant of ascorbic acid under specified condition. Figure 7 shows the oxidation of ascorbic acid at bare GC electrode and ZnO/GC modified electrode. The enhancement factor for low rotation rate is even better. At low rotation rate the current was controlled by transport of ZnO through the thick levich layer so that the behavior Ilim vs. ω1/2appeared to be linear. Since the electrode material used is different the potential of the oxidation ascorbic acid was observed to shift positively with increasing the electrode rotation rate. Based on the Kouteckey- Levich equation (3) below
1/il = 1/ik + 1/(0.62nFAω1/2 D0 2/3 V-1/6 C0 ) …(3)
Where ik is the kinetic current and w is rotation rate/angular velocity, the other parameters have its usual meaning. The value of diffusion coefficient was obtained by plotting a reciprocal graph of limiting current il versus square root of angular velocity, ω35. The diffusion coefficients obtained experimentally are 5.4 × 10-6 cm2s-1 and 5.7 × 10-6 cm2s-1 as shown in figure 8 for bare GC electrode and ZnO/GC modified electrode respectively. The rate constant for the catalytic reaction kf of the system were also calculated for both unmodified and modified GC using the equation (4) below:
ik= FAkf(E)C0* …(4)
where ik is the partition coefficient of the chemical reaction between ascorbic acid and redox sites of surface confined ZnO. The values of kinetic rate constant using the bare GC and ZnO/GC modified electrode are 2.5×10-3cms-1 and 2.1x 10-3cms-1 respectively. Difference between the kinetic rate constants is small but it is significant (19% increases in ks) as it implies the catalytic reaction become dominant with different electrode surface, which would invariably modify the electrochemical rate constant of the analyte.
Scanning Electron Microscopy (SEM)
Before electrolysis, ZnO appear rather scattered and powdery, penetrated with presence of agglomerate. Figure 9 (a) and (b) showed a more or less spherical shape and their average particle size is about 0.4 mm. As shown in Figure 9 (b), the size of particle agglomeration increased to crystallite size (9mm) diameter after electrolysis with the magnification of 3000 times.
Conclusions
ZnO/GC electrode was successfully fabricated and has exhibited electrocatalytic effect on the oxidation of ascorbic acid. The amount of ZnO microparticles were optimized, in which an important condition of the experiment is 0.2mg. The oxidation current of ascorbic acid at ZnO/GC modified electrode appeared with enhancement of 1.5 times compared to bare GC electrode. The modified electrode has been shown to increase the electrocatalytic oxidation current of ascorbic acid. From the hydrodynamic studies using rotation disk electrode, the values of kinetic rate constant and diffusion coefficient for ascorbic acid were found to be 5.7× 10-6 cm2s-1 and 2.1×10-3cms-1 respectively using ZnO/GC modified electrode.
Acknowledgments
The authors wish to thank University Putra Malaysia for providing research facility and financial support for the accomplishment of this work.
References
- M.H. Pournaghi-Azar, R. Sabzi, J. Electroanal. Chem. 543: 115 (2003).
- Hung-Wei Chu, R. Thangamuthu, Shen-Ming Chen Electrochimica Acta 53: 2862 (2008).
- W. Yantasee, Y. Lin, G.E. Fryxell, B.J. Busche, Anal. Chim. Acta 502: 207 (2004).
- M.H. Pournaghi-Azar, H. Dastangoo, J. Electroanal. Chem. 523: 26 (2002).
- S. Ashok Kumar, Hui-Wen Cheng, Shen-Ming Chen Reactive & Functional Polymers 69: 364 (2009).
- P.P. Sahay, R.K. Nath Sensors and Actuators B 133: 222 (2008).
- Rahman Hallaj, Abdollah Salimi, Keivan Akhtari, Saied Soltanian, Hussein Mamkhezria Sensors and Actuators B 135:632(2009).
- Nadezda Alexeyeva, Timo Laaksonen, Kyo sti Kontturi, Fakhradin Mirkhalaf, DavidJ.Schiffrin, Kaido Tammeveski, Electrochem. Commun. 8: 1475 (2006).
- Hui Fang Cui, Jian-Shan Ye, Wei-De Zhang, John Wang, Fwu-Shan Sheu, J. Electroanal. Chem. 577: 295 (2005).
- Liang Wang, Shaojun Guo, Lijian Huang, Shaojun Dong, Electrochem. Commun. 9:827 (2007).
- Guoyu Gao, Daojun Guo, Ce Wang, Hulin Li, Electrochem. Commun. 9: 1582 (2007).
- Keith B. Male, Sabahudin Hrapovic, Yali Liu, Dashan Wang, John H.T. Luong, Anal. Chimica Acta 516: 35 (2004).
- Sheng-Fu Wang, Fen Xie, Ruo-Fei Hu, Sensors and Actuators B 123: 495 (2007).
- Zifeng Deng, Yang Tian, Xia Yin, Qi Rui, Haiqing Liu, Yongping Luo, Electrochem. Commun. 10: 818 (2008) .
- S.R. Sivakkumar, Jang Myoun Ko, Dong Young Kim, B.C. Kim and G.G. Wallace, Electrochim. Acta 52: 7377 (2007).
- H.L. Pang, J.P. Lu, J.H. Chen, C.T. Huang, B. Liu and X.H. Zhang, Electrochim. Acta 54: 2610 (2009).
- Dong Fang, Kelong Huang, Shuqin Liu, Zhaojian Li Journal of Alloys and Compounds 464: L5–L9 (2008).
- R.W. Murray (Ed.), Molecular Design of Electrode Surfaces. Techniques of Chemistry Series, vol. XXII, Wiley, New York (1992).
- H. Razmi-Nerbin, M.H. Pournaghi-Azar, J. Solid State Electrochem. 6: 126 (2002).
- C.X. Cai, H.Y. Ju, H.Y. Chen, J. Electroanal. Chem. 397: 185 (1995).
- O. Ikeda, H. Yoneyama, J. Electroanal. Chem. 265: 323 (1989).
- M.H. Pournaghi-Azar, H. Razmi-Nerbin, J. Electroanal. Chem. 83: 456 (1998).
- M.H. Pournaghi-Azar, H. Nahalparvari Journal of Electroanalytical Chemistry 583: 307 (2005).
- D.M. Zhou, H.X. Ju, H.Y. Chen, J. Electroanal. Chem. 408: 219 (1996).
- S. Jayarama Reddy, A. Dostal, F. Scholz, J. Electroanal. Chem. 403: 209 (1996).
- G.Y. Shi, J.X. Lu, F. Xu,W.L. Sun, L.T. Jin, K. Yamamoto, S.G. Tao, J.J.Y. Jin, Anal. Chim. Acta 391: 307 (1999).
- S. Zhang,W.L. Sun,W. Zhang,W.Y. Qi, L.T. Jin, K. Yamamoto, S.G. Tao, J.Y. Jin, Anal. Chim. Acta 386: 21 (1999).
- W.T.Tan, J.K.Goh, Electroanalysis 20(22): 2447 (2008).
- J.K.Goh W.T.Tan, .F.Lim,and N.A.M.Maamor Analytical Sciences Malaysia.12(2): 480 (2008).
- Q. Lei, G. Qiang, S. Yonghai, L. Zhuang, Y. Xiurong, Sens. Actuators 107: 303 (2005).
- L. Hang, S. Dong, J. Electroanal. Chem. 568: 189 (2004).
- Z. Suxia, F. Yaqin, S. Changqing, Electroanalysis 15: 739 (2003).
- M. Shaolin, K. Jinqing, Synth. Met. 29: 132 (2002).
- W.T.Tan, A.M.Bond, S.W.Ngooi, E.B.Lim, J.K.Goh, Analytica Chimica Acta 491: 181 (2003).
- A.J.Bard, L.R.Faulker, Electrochemistry Methods, Fundamental and Application, Wiley, New York (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









