Biobed system to reduce four pesticide organophosphorus point contamination at farm level
A. Lemerhyeratte, M. Zougagh, O.Id El Mouden, R. Salghi, L. Bazzi, A. Hormatallah and S. Zine
1Laboratoire Régionale de l’Etablissement Autonome de Contrôle et de Coordination des Exportations d’Agadir, (Maroc).
2Laboratoire d’Ingénieries des Procédés de l’Energie et de l’Environnement, Ecole Nationale des Sciences Appliquées, B.P 1136 Agadir, (Maroc).
3Department of Analytical Chemistry and Food Technology, Faculty of Chemistry, University of Castilla-La Mancha, Av. Camilo José Cela s/n, E-13004 Ciudad Real, Spain
4Albacete Science and Technology Park. E-02006 Albacete, (Spain).
5Laboratoire Matériaux & environnement, Equipe de Chimie Physique Appliquées, Faculté des Sciences, BP 8106, Agadir (Morocco).
6Laboratoire des pesticides, Institut Agronomique et Vétérinaire Hassan II, Complexe Horticole d’Agadir, (Maroc). 7Ecole Supérieure de Technologie d’Agadir, B.P: 33/S Agadir.Article Received on :
Article Accepted on :
Article Published : 02 Mar 2010
A potential method for cleaning water from point-source pollution by organic compounds using biological reactors. In this study, five reactors were tested for their ability to retain and degrade pesticides. The pesticides tested were the insecticide chlorpyriphos ethyl (CE), malathion (MAL), dimethoate (DIM) and methamidophos (MET). The reactors were filled with differing mixtures of soil, bleaching earth and straw. The reactor volume was 10 L. Forced circulation of the contaminated solution was programmed to decontaminate the solution. Both retention and degradation of the compounds by the reactors was studied. The degradation rate of the pesticides in the reactors was fast. The reactors retained CE, MAL and DIM very strongly, and retained MET less strongly. The half-life of all pesticides in the reactors was less than 21 days, compared to literature values of 60–70 days in soil. The combined retention and fast degradation make the biofilter a feasible technique to reduce spill-related and point environmental contamination by pesticides. The technique is most effective against persistent pesticides, while for mobile pesticides, the efficiency can be improved with several passages of the contaminated solution through biofilters.
KEYWORDS:Biobeds; pesticides; soil; bleaching earth and straw
Download this article as:| Copy the following to cite this article: Lemerhyeratte A, Zougagh M, El Mouden O. Id, Salghi R, Bazzi L, Hormatallah A, Zine S. Biobed system to reduce four pesticide organophosphorus point contamination at farm level. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Lemerhyeratte A, Zougagh M, El Mouden O. Id, Salghi R, Bazzi L, Hormatallah A, Zine S. Biobed system to reduce four pesticide organophosphorus point contamination at farm level. Available from: http://www.orientjchem.org/?p=11494 |
Introduction
Contamination of water bodies with agricultural pesticides poses a significant threat to aquatic ecosystems and drinking water resources (Dabrowski and Schulz, 2003). A major point source of this contaminationis spills during filling and cleaning of spraying equipment. These activities often are performed at particular on-farm sites due to the convenience ofa water supply and high concentrations of pesticide residues have been found at such sites (Helweg, A, 1994). If spillages take place in a farmyard where the topsoil layer has been replaced by a layer of gravel and sand, there is an obvious risk of groundwater contamination from leaching. Several techniques have been developed for the removal of pesticides from water. Sorption on activated carbon is the most widespread technology used to deal with purification of water contaminated by pesticides and other hazardous chemicals (Baup et al., 2000; Heijman and Hopman, 1999). However, due to high cost of activated carbon, its use in the field is sometimes restricted due to economical considerations. Moreover, the high cost associated with its regeneration led to explore new inexpensive materials (Gupta et al., 2006). For the last decades, sorption of contaminants by sorbents of natural origin has gained important credibility due to the good performance and low cost of these complex materials (Bras et al., 1999; Chubar et al., 2003; Sheng et al., 2005; Yang and Sheng, 2003a,b). A technique which uses natural materials (agricultural waste products or products available on-farm) to create a pesticide retaining and degrading environment to clean contaminated water on-farm, has been developed in the first time in Sweden (Torstensson and Castillo, 1997; Torstensson, 2000) and was called a biobed. it consist to intercept and treat contaminated runoff from the farmyard and/ or drips and spillages arising during the filling process. Biobeds rely on a mixture of organic matter and soil as biofilters for retaining and biodegrading pesticides employed in agricultural crop protection (Torstensson and Castillo, 1997, Fogg et al., 2003a, Henriksen et al., 2003) and are effective in reducing point source contamination (Torstensson, 2000). The typical Swedish biomix consists of straw, topsoil, and peat (50-25-25% v/v). The straw stimulates the growth of lignin-degrading fungi and the activity of ligninolytic enzymes (such as manganese and lignin peroxidases), which can degrade many different pesticides (Castillo et al., 1997, 2000 2001a,b). The soil provides sorption capacity and other degrading microorganisms, and the peat contributes to high sorption capacity and also regulates the humidity of the system. A grass layer covering the biobed helps to keep the correct humidity and can be used as an indicator to reveal pesticide spills. To adapt this biological system to Mediterranean operating conditions, there is a need to identify organic materials that can act in the same effective way as those in the original Swedish composition. The type of organic material present in the biobed is crucial for the amount, activity, and genotypic and phenotypic versatility of microorganisms responsible for degradation of pesticides and their metabolites. Studies have demonstrated that biobeds can effectively retain and degrade pesticides (Rose et al., 2003, Torstensson, 2000, Fogg et al., 2003a,b, Henriksen et al., 2003), such that the concentrations of pesticides being released from the farmyard are significantly reduced.
The aim of this work is to evaluate the degradation capacity of an organic mixture constituted of bleaching earth, straw and soil against four pesticides such as chlorpyriphos ethyl, malathion, methamidophos and dimethoate. These pesticides were considered as test substances because they are widely used citrus crop system in Morocco and characterized by different physico-chemical characteristics and different microbial metabolism. In particular, the influence of several factors such as initial concentration, co-application and repeated applications on these pesticides degradation was investigated in order to ascertain the suitability of the organic mixture tested to be used as a biofilter in biobed systems.
Material and Methods
Chemicals
Chlorpyriphos Ethyl (O,O–diethyl O-3,5,6 trichloro-2-pyridylphosphorothioate), Malathion (Diethyl (dimethoxy-thio-phosphoryl-thio) succinate), Methamidophos (O,S-Dimethyl-phosphor-amido-thioate) and dimethoate (O,O-dimethyl S-methylcarbamoyl methyl phosphoro-dithioate) were supplied by Dr. Ehrenstorfer GmbH (Augsburg, Germany).The active substance, chemical name, molecular weight, hemical formula and commercial name of each formulation are shown in Table 1. The organic solvents were obtained from Panreac (Barcelona, Spain). Each standard was dissolved in acetone to obtain stock solutions of approximately 200 mg/L, which were stored light protected at 4 ºC until further use. The freshly working standard solutions were obtained by dilutions with n-hexane. Florisil adsorbent (16– 30 mesh) was obtained from Sigma–Aldrich (St. LOUIS, MO, USA).
Biofilters and Reactors
Five biofilters were prepared with farm-available materials: Soil, bleaching earth and straw in different percent. The composition of this biofilters is reported in the Table 2.
The five different mixtures (1,05 kg) were placed in confined tin reactors of 10 l volume after a conditioning period of 15 days, useful to give a mixing of different materials. An additional reactor with no biofilter was used as a reference. The reference and measurement reactors are shown in Figure 1. Daily, 5 l of water (based on volumes of waster likely to be generated on a farm) was washed through the reactor using a peristaltic pump with 0.3 L min-1 flow rate (taking 16.67 min). The water was previously contaminated with 0.3 g L-1 of each of the four pesticides used in this study. The four pesticides were used in actual citrus crop system in Morocco and the doses are related to normal agricultural applications. The main experiment continued for six weeks. Weekly, five 50 ml of water samples were collected from each reactor and analyzed for the four pesticides. Every two weeks 20 g samples of solid organic materials were collected from biofilters and analyzed for the four pesticides.
Instruments apparatus and chromatographic conditions
Analysis of the all pesticides were carried out with a Hewlett–Packard 6890 gas chromatograph equipped with an NPD Detector, on column injection port, and fused silica capillary column HP-5 column (5% diphenyl and 95% dimethylpolysiloxane, 25 m ´ 0.32 mm ID, 0.52 µm film thickness); and temperature programming from 80ºC to 160ºC (25ºC/min), 160-220 °C at 10°C/min. 220 – 240 °C at 10°C/min, 80 °C (3.00 min), 160 °C (2.00 min), 220 °C (10.00 min), 240 °C (8.80 min) ; injector temperature 73ºC to 250ºC (180ºC/ min); and detector temperature 300ºC. Carrier gas (helium) flow rate, 2.6 mL/min; makeup gas (nitrogen) flow rate, 10 mL/min; Air 60 ml/min; H2 3 ml/min. injection volume, 1 µl; Data were acquired and the equipment controlled by using HP Chem-Station software, which was run under Microsoft Windows NT on an hp compatible personal computer. The identification of the a.i. peaks and the absence of interfering substances were assessed by comparing the sample chromatograms with those of a pesticide standard mixture and of an untreated sample. The internal standard method was used to quantify the residues by measuring peak areas vs. concentrations.
Pesticide extraction and analysis
The method used for the extraction of pesticide on water and soil was adapted from Charles and Raymond (Charles et al., 1991). For each 5 g of soil or 1 ml of water, 150 ml of acetone was added and the mixture washomogenised for 2 minutes and after mixedduring 2 hours. The mixture was filtered with glass wool. After filtration, the acetone residues were partitioned with saturated aqueous sodium chloride (30 ml) and dichloromethane (70 ml) in a separating funnel. The extraction was repeated with other 70 ml of dichloromethane and dried over anhydrous sodium sulphate. The dichloromethane fraction was collected and evaporated on a rotatory vacuum evaporator at 40°C and the residues were dissolved in hexane (5 ml). The eluate was evaporated to dryness under a stream of nitrogen and reconstituted in 1 ml of hexane, and this solution was injected into the GC system.
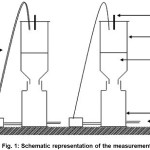 |
Figure 1: Schematic representation of the measurement Click here to View figure |
 |
Figure 2: Concentration of the four pesticides in water of the reference and measurement Click here to View figure |
 |
Figure 3: Degradation curves of the four pesticides in the five substrates (mixture 1, 2 , 3, 4 and 5) Click here to View figure |
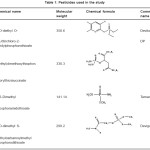 |
Table 1: Pesticides used in the study Click here to View table |
extraction was repeated with other 70 ml of dichloromethane and dried over anhydrous sodium sulphate. The dichloromethane fraction was collected and evaporated on a rotatory vacuum evaporator at 40°C and the residues were dissolved in hexane (5 ml). The eluate was evaporated to dryness under a stream of nitrogen and reconstituted in 1 ml of hexane, and this solution was injected into the GC system.
Results and Discussion
Figure 2 shows the change in concentration of the four pesticides in the water of the reference and measurement reactors. The quote of pesticide decay due to temperature effect and photodecomposition and volatilization is very slight or completely absent, looking at curves of reference reactors. Decay curves in measurement reactors show a very rapid disappearance of CE, MAL and DIM from water of all reactors, mainly due to the immediate and very strong adsorption on biofilters, and a moderate disappearance of METI from all mixtures, indicating a low depuration efficiency of these biofilters for this pesticide. The effect of the three composts is clear for MET in organic material used in biofilters studied. At week 6 more than 40% of MET remained in water, while water was clean for three others pesticides studied in all mixtures used in biofilters studied.
Table 2: Composition of organic materials used in biofilters.
| Mixture | Weight of soil (g) | Weight of bleaching earth (g) | Weight of straw (g) |
| 1 | 300 | 700 | 50 |
| 2 | 400 | 600 | 50 |
| 3 | 500 | 500 | 50 |
| 4 | 600 | 400 | 50 |
| 5 | 700 | 300 | 50 |
It is important to know what happened in the biofilter, once the pesticides were retained in order to evaluate not only their retention capacity, but also their degradation ability. Degradation curves in biofilters are reported in Figure 3. The start of the curves (100%) represents the amount of pesticides in biofilters after the first washing cycle which is different depending on substrate and pesticide. In spite of long half-life of the four pesticides in standard soils (Table 1), the half-life values in biofilters varied from 9 to 12 days for CE, while MAL, DIM and MET disappeared after 21 days. These results indicate that degradation in the biofilter appears to be fast enough to allow a decontamination of the substrates occurring a few weeks after the beginning of the experiment, with the possibility of re-utilizing the materials more than once or for agricultural uses, such as amendments, and to avoid the presence of pesticides in the environment.
Conclusions
The reactor technique presented here has proved to be veryefficient for the cleaning of water contaminated with pesticides. The degradation rate of the pesticides in the reactors was fast. The reactors retained CE, MAL and DIM very strongly, and retained MET less strongly. When applied at the field scale, polluted water would pass several times through the filter, allowing even poorly retained pesticides time to degrade. Therefore the biomassbed proposed could help in reducing the pesticide point contamination at farm level.
Acknowledgements
The authors wish to thank the NATO program (CBP.MD.CLG 983108) for supporting this Work. The Spanish Ministry of Education and Science and JJCC Castilla-La Mancha are gratefully acknowledged for funding this work with Grants CTQ2007-61830 and PCC08-0015-0722, respectively. The support given through a ‘‘INCRECYT’’ research contract to M. Zougagh is also acknowledged.
- Baup, S., Jaffre, C., Wolbert, D., Laplanche, A., Adsorption of pesticides onto granular activated carbon: determination of surface diffusivities using simple batch experiments. Adsorption 6: 219–228 (2000).
- Bras, I.P., Santos, L., Alves, A., Organochlorine pesticides removal by pinus bark sorption. Environ. Sci. Technol. 33: 631–634 (1999).
- Chubar, N., Carvalho, J.R., Correia, M.J.N., Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II). Colloid Surf. A 230: 57–65 (2003).
- Castillo, M. d. P.; Ander, P.; Stenstro¨m, J. Lignin and manganese peroxidase activity in extracts from straw solid substrate fermentations. Biotechnol. Tech. 11, 701-706 (1997).
- Castillo, M. d. P.; Ander, P.; Stenstro¨m, J.; Torstensson, L. Degradation of the herbicide bentazon as related to enzyme production by Phanerochaete chrysosporium in a solid substrate fermentation system. World J. Microbiol. Biotechnol. 16: 289-295 (2000).
- Castillo, M. d. P.; von Wiren-Lehr, S.; Scheunert, I.; Torstensson, L. Degradation of isoproturon by the white rot fungi Phanerochaete chrysosporium. Biol. Fertil. Soils 33: 521-528 (2001a).
- Castillo, M. d. P.; Andersson, A.; Ander, P.; Stenstrom, J.; Torstensson, L. Establishment of the white rot fungus Phanerochaete chrysoporium on unsterile straw of solid substrate fermentation systems intended for degradation of pesticides. World J. Microbiol. Biotechnol. 17: 627-633 (2001b).
- Dabrowski, J.M., Schulz, R., Predicted and measured levels of azinphosmethyl in the Lourens River, South Africa: Comparison of runoff and spray drift. Environ. Toxicol. Chem. 22: 494–500 (2003).
- Fogg, P.; Boxall, A. B. A.; Walker, A.; Jukes, Pesticide degradation in a “biobed” composting substrate. Pestic. Manage. Sci. 59: 527-537 (2003a).
- Fogg, P.; Boxall, A. B. A.; Walker, A. Degradation of pesticides in biobeds: The effect of concentration and pesticide mixtures. J. Agric. Food. Chem. 51: 5344-5349 (2003b).
- Gupta, V.K., Ali, I., Suhas, Saini, V.K., Adsorption of 2, 4-D and carbofuran pesticides using fertilizer and steel industry wastes. J. Coll. Interf. Sci. 299: 556– 563 (2006).
- Heijman, S.G.J., Hopman, R., Activated carbon filtration in drinking water production: model prediction and new concepts. Colloid Surf. A 151: 303–310 (1999).
- Helweg, A. Threats to water quality from pesticidessCase histories from Denmark. Pesticide Outlook 5: 12-18 (1994).
- Henriksen, V. V.; Helweg, A.; Spliid, N. H.; Felding, G.; Stenvang, L. Capacity of model biobeds to retain and degrade mecoprop and isoproturon. Pestic. Manage. Sci. 59: 1076- 1082 (2003).
- Rose, S. C.; Basford, W. D.; Carter, A. D. On-farm bioremediation systems to limit point source pesticide pollution. Proceedings of the XII Symposium on Pesticides and Chemicals, 04-06 June, Piacenza, Italy, 559-566 (2003).
- Sheng, G.Y., Yang, Y.N., Huang, M.S., Yang, K., Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ. Pollut. 134: 457–463 (2005).
- Torstensson, L., Castillo, M.D.P., Use of biobeds in Sweden to minimize environmental spillages from agricultural spraying equipment. Pestic. Outlook 8: 24-27 (1997).
- Torstensson, L., Experiences of biobeds in practical use in Sweden. Pesticide Outlook 10: 206–211 (2000).
- Yang, Y.N., Sheng, G.Y., Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ. Sci. Technol. 37: 3635-3639 (2003a).
- Yang, Y.N., Sheng, G.Y., Pesticide adsorptivity of aged particulate matter arising from crop residue burns. J. Agric. Food Chem. 51: 5047–5051 (2003b).

This work is licensed under a Creative Commons Attribution 4.0 International License.









