Aldol condensation of 2,5-dimethoxybenzaldehyde with actone under basic conditions
Siti Aminah MOHD. Shah1, Faujan Bin Hj. Ahmad1* and MOHD.Rashidi Abdull Ma
¹Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor (Malaysia).
Article Received on :
Article Accepted on :
Article Published : 02 Mar 2010
Mixed aldol condensation of 2,5-dimethoxybenzaldehyde and acetone with mole ratio of 2:1 in the presence of sodium hydroxide gave an orange-yellowish crystal; identified as 1,5-Bis(22 ,52 - dimethoxyphenyl)penta-1,4-diene-3-one yield 56 to 82 %.
KEYWORDS:Aldol Condensation; Curcumin Derivatives; 1,5-Bis (22 ,52 -Dimethoxyphenyl) Penta-1; 4-Diene-3-One
Download this article as:| Copy the following to cite this article: Shah S. A. M, Ahmad F. B. HJ, Ma M. R. A. Aldol condensation of 2,5-dimethoxybenzaldehyde with actone under basic conditions. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Shah S. A. M, Ahmad F. B. HJ, Ma M. R. A. Aldol condensation of 2,5-dimethoxybenzaldehyde with actone under basic conditions. Available from: http://www.orientjchem.org/?p=11491 |
Introduction
The aldol condensation is an important reaction in C-C bond forming1:
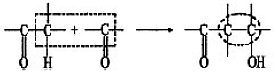
When catalyzed by base, the first step is the abstraction of a α-proton from an aldehyde or ketone. Then, the carbanion formed attacks the carbonyl group of another aldehyde or ketone molecule to obtain a β-hydroxy aldehyde or ketone (aldol) and can be easily dehydrated in situ to α,β-unsaturated carbonyl compounds. This reaction is commonly used to manufacture solvents, plasticizers and intermediates for the manufacture of perfumes and pharmaceuticals2.
Curcumin, (1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione is a naturally occurring phenolic compound isolated as yellow pigment from turmeric (curcuma longa) and occur as a water soluble powder3. This compound has received much attention due to its diversity of biological and pharmacological activities including antiinflammatory, antioxidant, antiviral, anti-HIV-1 integrase, chemo-preventive, anticancer and anti-Alzheimer’s effects reported in literature. It has entered the phase I clinical trials for chemoprevention conducted by National Cancer Institute, USA 4.
During the last decade, the synthesis of curcumin derivatives or its analogues has been an attracting research area for many groups of researches. Mukhopadhyay et al., have studied the anti-inflammatory activity of curcumin (C), diethyl curcumin (DAC), triethyl curcumin (TEC),tetrahydrocurcumin (THC), and phenylbutazone
(PB) by using carrageenin-induced rat paw oedema. These curcumin-analogues showed both anti-inflammatory (protective) and inflammation-increasing (irrititant) effects. The rank of order of potencies of curcumin analogues and PB in the carrageenin-induced inflammation is; THC > C > PB > TEC, whereas DAC is devoid of anti-inflammatory activity 5.
Mishra et al.,6 have studied the structure correlation for the anti-malarial activity of curcumin analogous. They reported that, pyrazole curcumin, 3-nitrophenylpyrazole curcumin, and 4-hydroxy-3-methoxy-benzylidene derivatives were more potent than curcumin itself. These compounds inhibited at nano-molar concentration. Their enhanced potencies were probably due to their increased cellular uptake and/ or retention by the parasite in addition to their greater efficacies of inhibiting the target itself.
Thus, we report there in the reaction of 2,5-dimethoxybenzaldehyde with acetone to give a curcumine derivative in 60-82 % yield, depending on the reaction condition.
Experimental
The IR spectrum was recorded on Perkin-Elmer FTIR Model Spectrum BX spectrophotometer, while MS spectrum was recorded on Shimadzu model QP5050A Gas Chromatography Mass Spectrometer, and NMR was recorded on JOEL FTNMR 400 MHz-transformed spectrometer. Melting points were checked and were determined using digital melting apparatus, IA9000 series while the percentage of carbon and hydrogen were checked by using CHNS-932 Elemental Analyzer.
Reaction at Room Temperature
A mixture of 2,5-dimethoxybenzaldehyde (830 mg, 5.0 mmol), acetone (0.2 ml, 2.5 mmol), ethanol (10 ml) and sodium hydroxide (20 ml, 1.0 M) was stirred at room temperature for 2 hours. The yellowish crystal was then filtered off and dried to give desired curcumin derivative. Crystallization from ethanol gave a yellow crystal (490 mg, 56%) and melted at 102 – 105 °C. It had υmax cm-1 3436 (O-H), 2918 and 2848 (C-H stretching), 1648 (C=C alkene), 1606 (C=O), 1496 (C=C aromatic), 1286 (C=O bending), 1222, 1178 and 1114 (C-O stretching). 1H NMR (400 MHz, CDCl3 5%) δ, 3.82, 3.87 (each 3H, s, 2′-OCH3, 5′-OCH3), 6.88 (1H, d, J = 6Hz, H-4′), 8.01 (1H, d, J = 6 Hz, H-3′), 6.93, 6.95 (each 1H, dd, J = 16 Hz), 7.16 (1H, s, H-6′). ä13C NMR, 55.82, 56.11 (each for 2′-OCH3, 5′-OCH3), 112.43, 153.50 (each for C-4 and C-5 ), 189.85
(C=O). m/z: 354 for C21H22O5. There were required C, 71.19 % and H, 6.21 % as calculated. From CHNS analysis, it found that C is 72.23% while H is 6.16%.
Reaction at Refluxing Temperature
The reaction was carried out by reacting a mixture of 2,5-dimethoxybenzaldehyde (830 mg, 5.0 mmol), acetone (0.2 ml, 2.5 mmol), ethanol (10 ml) and sodium hydroxide (20 ml, 1.0 M). The mixture was refluxed for 2 hours. The crystal was then filtered off and dried to give desired curcumin derivative (720 mg, 82 %). It had similar spectroscopic data as above in experiment (a).
Results and Discussion
Mixed aldol condensation was common for creating new compounds containing carbonyl groups. The above reaction was carried out at room temperature (30 °C) and refluxed temperature, giving the expected product in 56 % and 82 % yield respectively.
The IR spectrum of this compound showed broad signal at υmax 3436 cm-1 suggesting the presence of hydroxyl group, signals at υmax 2918 and 2848 cm-1 were due to stretching of C-H bonding of CH, CH2 or CH3 groups, respectively. The signal at υmax 1648 cm-1 showed the presence of alkene double bond C=C and the C=O stretching was assigned at υmax 1606 cm-1 while at υmax 1496 cm-1 showed the presence of C=C aromatic peak. The signal at υmax 1286 cm-1 assignd for C=O bending, at υmax 1222, 1178 and 1114 cm-1 were assigned for the presence of C-O stretching, corresponded to – OCH3 group. The signal in the range of υmax 990 to714 cm-1 was assigned to the presence of para substitution of benzene ring.
Table 1: 1H NMR (400 MHz CDCl3), assignments and COSY Correlation for 1,5- Bis(22, 52 – dimethoxyphenyl)penta-1,4-diene-3-one
| H Number | d (ppm) | COSY Correlation | |
| H-1 | 6.93 | (dd, 1H) | H-2, H-4′, H-6′ |
| H-2 | 6.95 | (dd, 1H) | H-1, H-4′, H-6′ |
| H-3′ | 8.01 | (d, 1H) | H-6′ |
| H-4′ | 6.88 | (d, 1H) | H-1, H-2 |
| H-6′ | 7.17 | (s, 1H) | H-1, H-2, H-3′ |
| 2′-OCH3 | 3.82 | (s, 3H) | – |
| 5′-OCH3 | 3.87 | (s, 3H) | – |
Table-2: 13C NMR, 1H NMR, HMBC and HMQC assignments for 1,5-Bis (22 ,52 -dimethoxyphenyl)penta-1,4-diene-3-one
| Carbon no. | d 13C (in CDCl3) | 1H | HMBC | HMQC |
| 1 | 113.15 | 6.93 | H-1, H-2, H-3′, H-4′ | H-1 |
| 2 | 117.18 | 6.95 | H-4′, H-6′ | H-2 |
| 3 | 189.85 | – | H-3′, H-6′ | – |
| 4 | 117.18 | 6.95 | H-4′, H-6′ | H-4 |
| 5 | 113.15 | 6.93 | H-1, H-2, H-3′, H-4′ | H-5 |
| 1′ | 124.50 | – | H-4′, H-6′ | – |
| 2′ | 153.51 | – | OCH3 (C-2′), OCH3 (C-5′), H-1, H-2, H-3′, H-4′, H-6′ | – |
| 3′ | 138.05 | 8.01 | H-4′, H-6′ | H-3′ |
| 4′ | 112.43 | 6.88 | H-3′ | H-4′ |
| 5′ | 153.10 | – | OCH3 (C-2′), OCH3 (C-5′), H-1, H-2, H-3′, H-4′, H-6′ | – |
| 6′ | 126.32 | 7.16 | H-1, H-2, H-3′, H-4′ | H-6′ |
| 2′-OCH3 | 56.11 | – | – | – |
| 5′-OCH3 | 55.82 | – | – | – |
It’s 1H NMR signals (400 MHz) in CDCl3 resonated at ä 3.82 (s, 3H), 3.87 (s, 3H) corresponded to the two methoxy groups and assigned to C-2′ and C-5′ of the structure. Signal at ä 6.88 (d, J = 6 Hz, 1H) and ä 8.01 (d, J = 6 Hz, 1H) were corresponded to the aromatic ring of H-4′ and H-3′. A singlet appeared at ä 7.16 was assigned to H-6′ of the aromatic. There were two pairs doublet of doublet signals with J = 16 Hz appeared which corresponded to C=C bond. These peaks were assigned to H-1 and H-2 which appeared at ä 6.93 and 6.95 at trans position.
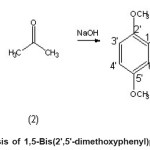 |
Scheme 1: Synthesis of 1,5-Bis(2′,5′-dimethoxyphenyl)penta-1,4-diene-3-one Click here to View scheme |
The 13C NMR spectrum was accounted for 11 carbons signal at ä 55.82 and ä 56.11 assigned the carbons of two methoxy groups; signals at ä 112.43 and 153.50 were corresponded to the two carbon-double bonds, while single signal at very down field, ä 189.85 was assigned for carbonyl carbon. The DEPT spectrum shows the presence of five CH groups and two methoxy groups. This deduced structure was clearly supported by the COSY (Table 1), HMQC and HMBC (Table 2) spectra. All of these results confirmed the structure of 1,5-Bis(22 ,52 -dimethoxyphenyl)penta-1,4-diene-3-oneThe synthesis pathway is shown in scheme 1:
Conclusion
1,5-Bis(2′,5′-Dimethoxyphenyl) penta-1,4-diene-3-one a derivative of curcumin was obtained from the reaction of 2,2-dimethoxybenzaldehyde and acetone in the present of sodium hydroxide as the base.
References
- McMurry J., Organic Chemistry, 7th edition, Thomson Learning Brooks/Cole, Washington 877-888 (2008).
- Veloso, C.O., Henriques, C.A., Dias, A.G., Monteiro, J.L.F., Catalysis Today, 107-108: 294-301 (2005).
- Liang, S., Hong, F.J., Spectrochimica Acta Part A, 67: 619–623 (2007).
- Zhang, Q., Fu, Y., Wang, H.W., Gong, T., Qin, Y., Zhang, Z.R., Chinese Chemical Letters, 19: 281-285 (2008).
- Mukhopadhyay, M.J.; Saha, A., Mukherjee, A. Food. Chem.Toxicology, 36: 73-76 (1998).
- Mishra, S., Karmodiya, K., Suroliab, N., Surolia, A. Bioorganic & Medicinal Chemistry, 16: 2894-2902 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









