A one-pot electro oxidative nuclear and side chain acetoxylation of aromatic compounds at platinum electrode
Manish K. Srivastav, Apoorv Saraswat and R. K. P. Singh*
Department of Chemistry, University of Allahabad, Allahabad - 211 002 (India)
Article Received on :
Article Accepted on :
Article Published : 01 Mar 2010
A direct, one-pot electrooxidative acetoxylation of aromatic compounds is reported here. Preparative electrolysis of various aromatic compounds was studied in the presence of acetic acid using DMF or acetonitrle as a non-aqueous solvent and suitable supporting electrolytes. The controlled potential electrolysis of aromatic compounds afforded nuclear and side chain acetoxylation depending upon the nature of the solvent and supporting electrolytes. A plausible mechanism for the acetoxylation process is discussed.
KEYWORDS:Pt-electrode; supporting electrolyte; electrochemical oxidation; green chemistry
Download this article as:| Copy the following to cite this article: Srivastav M. K, Saraswat A, Singh R. K. P. A one-pot electro oxidative nuclear and side chain acetoxylation of aromatic compounds at platinum electrode. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Srivastav M. K, Saraswat A, Singh R. K. P. A one-pot electro oxidative nuclear and side chain acetoxylation of aromatic compounds at platinum electrode. Available from: http://www.orientjchem.org/?p=11512 |
Introduction
Electrochemical routes for organic synthesis must compete with more traditional chemical methods, as well as with emerging methods (e.g., enzymatic catalysis, photocatalysis). In view of environmental mandate, there is a global effort to replace the conventional catalysts by eco-friendly catalysts. Recently, extensive research work has been carried out on the procedure of esterification of acid with alcohol using catalysts such as zeolitic molecular sieves1, ion-exchange resin2, zirconium sulfate3, hafnium or zirconium salt4, polymer supported titanium tetrachloride4, ZrO2 and Mo-ZrO2 solid acid catalysts5 and heteropoly acids6 etc. Most of the reported procedures for the synthesis of esters require the use of sulfuric acid, hydrochloric acid and toxic chemicals like dimethyl sulfate, methyl iodide or unsafe reagents such as diazomethanes which are environmentally hazardous and unacceptable.
The electroorganic synthesis has certain merits because these reactions do not require oxidizing chemicals. It involves application of a potential in the presence of active electrode surfaces and the resulting flow of current drives the oxidation or reduction and subsequent recombination of reactants7. Electrochemistry can be considered a green methodology in organic synthesis, due to its non-polluting reagent, the electron8. At constant potential, a variety of electrochemical reactions takes place when a solution containing a reacting material is oxidized or reduced which depend on the structure of reacting molecules and the environment viz. nature of solvent, value of electrode potential and supporting electrolytes. After some preliminary discussion it is clear that one or other electrochemical processes can be making favorable by adjusting experimental parameters9. In the present communication we are reporting the result of our comparative study on the electrochemical nuclear and side chain acetoxylation of some aromatics viz. benzene, toluene, ethyl benzene, anisole, naphthalene, anthracene etc.
Results and Discussion
In any electrochemical study, the first question which must be answered is which component of the solution is actually undergoing electron transfer because the direct electrochemical oxidations or reductions of substrates utilize practically mass-free electrons as the only reagent10. During electrochemical acetoxylation, voltametric data shows that aromatic rings are more easily oxidized than acetate ion11. Acetoxylation of aromatic substrates has often been cited as evidence for the existence of the acetoxy radicals in the Kolbe reaction12,13. Recent studies have demonstrated that acetoxy radicals are intermediates in the Kolbe synthesis, but not in the formation of ring-substituted aromatic acetates14. Aromatic compounds are generally discharged below the critical potential for oxidation of acetate ion (1.90- 2.10 V vs SCE) and undergo what appears to be an electrophilic substitution reaction. In homologous solution, the lifetime of the acetoxy radical (10-9 – 10-10 sec) is estimated to be too short for reaction outside of the solvent cage in which it is formed 15.
In this paper, we discussed in details a selective one-pot electrochemical acetoxylation of our model aromatic compounds, naphthalene (A) and toluene (B), at room temperature in acetic acid and acetonitrile under various experimental conditions, affording 1-acetoxynaphthalene (C) and benzyl acetate (D) as the exclusive product. At room temperature, the electrooxidative acetoxylation of aromatic systems was carried out with sodium acetate or lithium perchlorate in acetic acid via an EC type mechanism at potential range from 1.25V to 1.95V. Most anodic reactions of aromatic compounds are initiated by oxidation of hydrocarbon, it is then appropriate to inquire into the nature of the oxidation step. It is generally accepted that most oxidations of aromatic hydrocarbons involve removal of one electron from the hydrocarbon to afford a radical cation. The structure of the radical cation and the environment in which it is created both influence its subsequent reactions. When the nucleophile is another reactant, it can attack the ring to afford a cationic intermediate after a second electron transfer, which most commonly will lose a proton to regenerate the aromatic ring and afford a nuclear substitution product.
Figure Electrochemical oxidation of aromatic ring at Pt-electrode
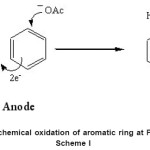 |
Scheme I Click here to View Scheme |
Indeed the electrochemical oxidation of alkyl aromatics in acetic acid affords clean side-chain substitution when a non-nucleophilic salt is used as the supporting electrolyte, whereas a mixture of side-chain and ring substitution products is obtained when sodium acetate is used as the supporting electrolyte. Nuclear substitution is favoured in the presence of good nucleophile and where the ring is not alkylated. The electrooxidative acetoxylation of aromatic nuclear ring was done by electrogenerated intermediate viz. radical cation. The proposed mechanism of this electrochemical reaction can be explained on the basis of attachment of acetoxy group on aromatic ring through the electrochemically generated radical cation of aromatic ring (Scheme II). The possible mechanism of electrooxidative nuclear ring acetoxylation of aromatic substrates is as follows:
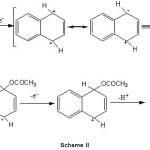 |
Scheme II Click here to View Scheme |
Electrochemical oxidation of toluene and ethyl benzene gives side-chain substituted acetoxylated products (6, 7 and 8) when lithium perchlorate or potassium nitrate is used as the supporting electrolyte in acetic acid as a solvent. The possible mechanism of electrooxidative side-chain acetoxylation of aromatic substrates is as follows:
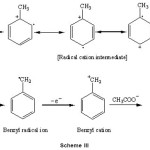 |
Scheme III Click here to View Scheme |
Single nuclear ring viz. benzene, naphthalene, anthracene in acetic acid affords the formation of nuclear substituted products when sodium acetate is used as a supporting electrolyte. In case of substituted aromatic ring, when lithium perchlorate and other supporting electrolytes are used, only side chain substituted products are obtained. In case of acetoxylation of polynuclear aromatic ring, the main products are nuclear substituted acetoxy aromatic ring. G. Bookmain and H. P. Frintz16 reported nuclear methoxylation of substrates possessing rather high oxidation potential is achieved with difficulty whereas naphthalene and anthracene are readily methoxylated. Electrochemically nuclear substitution in aromatic compounds is almost always preferred, which is reasonable since loss of a proton restored the aromatic stability and therefore has a low activation energy17. These results suggest that the substrate undergoes electron transfer to form a cationic species which reacts with acetate ion. Both cation radical and dication mechanisms in addition to a concerted two electron transfer to the anode followed by C-O bond formation have been proposed18.
Table 1: Current-potential data as recorded by potential cum galvenostat
| Name of Substrates | P* (V) | C* (mA) | Product | Y* (%) | Reagent/ Solvent | Supporting Electrolytes |
| Benzene | 1.75 | 11.9 | Phenyl acetate | 43 | Acetic acid | Sodium acetate |
| Toluene | 1.95 | 22.5 | Tolyl acetate | 34 | Acetic acid | Sodium acetate |
| Naphthalene | 1.75 | 8.3 | 1-Naphthyl acetate | 62 | Acetic acid, DMF | Lithium perchlorate |
| Naphthalene | 1.85 | 18.1 | 1-Naphthyl acetate | 72 | Acetic acid | Sodium acetate |
| Anisole | 1.65 | 12.7 | o, p- acetoxy anisole | 23 | Acetic acid | Sodium acetate |
| Anisole | 1.85 | 16.8 | Anisyl benzoate | 53 | Benzoic acid, AN | Tetraethyl amm. tosylate |
| Anthracene | 1.30 | 11.3 | 9-Acetoxyanthracene | 59 | Acetic acid, DMF | Lithium perchlorate |
| Toluene | 1.90 | 19.7 | Benzyl acetate | 22 | Acetic acid, AN | Lithium perchlorate |
| Toluene | 1.95 | 21.3 | Benzyl benzoate | 46 | Benzoic acid, AN | Lithium perchlorate |
| Ethyl benzene | 1.90 | 16.6 | Phenyl ethyl acetate | 27 | Acetic acid, AN | Lithium perchlorate |
P*= Potential in Volt, C*= Current in mA, Y*= Yield in percentage and AN= Acetonitrile
Significantly anodic acetoxylation does not occur with negatively substituted aromatics whose half-wave potentials are more anodic in value than the discharge of acetate ion. In case of acetoxylation of naphthalene with acetic acid in DMF or acetonitrile and lithium perchlorate or potassium nitrate as a supporting electrolyte, 1- acetoxy naphthalene was obtained with 1-methyl naphthalene also as a side product. The formation of 1-methylnaphthalene and generally, of methylated products from the anodic oxidations of certain aromatic compounds in HOAc-NaOAc has also been observed by Ross, Finkelstein, and Petersen and was explained as a result of the homolytic methylation of naphthalene by anodically generated methyl radical.
Both were separated by coloumn chromatography. The methylation of naphthalene is suppressed in favour of the acetoxylation process by operating at relatively low anode potentials. 9-Acetoxy anthracene was obtained on electrolysis of anthracene with acetic acid in DMF as a non aqueous solvent and lithium perchlorate as a supporting electrolyte. 9-Acetoxy-9,10-dihydroanthracene was also obtained as a side product.
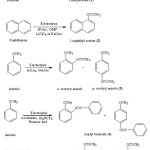 |
Figure 1: Electrooxidative nuclear ring acetoxylation of aromatic systems Click here to View figure |
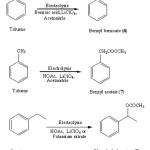 |
Figure 2: Electrooxidative side chain acetoxylation of aromatic systems Click here to View figure |
Experimental
Apparatus and reagents
The electrolysis was carried out at constant potential in four necked 50 ml undivided cell. For constant potential electrolysis we have used conventional three electrode undivided cell assembly designed in our laboratory using 1cm x 0.5cm platinum electrode19 as working as well as counter electrode. The platinum electrodes were surrounded from one side with glass material and saturated calomel electrode20 (SCE) is used as reference electrode to determine the anode potential. All chemicals are reagent-grade materials from Merck. These chemicals were used without further purification.
Preparation of reaction mixture and electroorganic synthesis of acetoxylated products
The reaction mixture for electrooxidative acetoxylation of various aromatic systems was prepared by us in two different routes. In first one we used 0.1 mol of aromatic substrate in 50 mL of acetic acid with 0.1 – 0.2 M sodium acetate as a supporting electrolyte simply and in second one, reaction mixture contains 0.1 mol of aromatic substrate in 50 mL of solution containing 10 mL acetic acid using 40 mL of acetonitrile or DMF as a nonaqueous solvent. Acetonitrile is the choice of solvent because it is resistance and unreactive with intermediate cation radical species21 in electrooxidative reactions. As a supporting electrolyte, we used 0.1M lithium perchlorate, potassium nitrate separately for each electrolysis. The working electrode potential was maintained between 1.20 and 1.95 V vs SCE. All the electrolysis was carried out at the oxidation potentials of their corresponding aromatic substrates.
At the initial stage, the reaction mixture was transparent and colourless. As electrolysis proceeded the platinum cathode surface was covered with dark yellowish layer of the product which continuously diffused in the bulk into the solution and the whole reaction mixture gets darkened. After terminating the reaction, most of the acetic acid was evaporated in a rotavapour at 40 °C. The product was extracted with water and ether system. After extraction, the solution was washed with sodium bicarbonate solution and water and drying with anhydrous magnesium sulfate. The distillation process was used to remove the ether and then residue was worked up using column chromatography on silica gel.
All the products were analyzed by chemical method as well as spectral technique. Mps were determined in open capillary tubes using Toshniwal’s melting point apparatus and are uncorrected. Compounds were purified by column chromatography. ¹H NMR (CDCl3) spectra were recorded on a Bruker AVANCE II-400 (400 MHz FT NMR) spectrophotometer using TMS as internal standard; IR (KBr) spectra on Perkin-Elmer 1800 and Shimadzu 8201 PC (4000-350 cm-1) FTIR spectrophotometer; mass spectra on Jeol D-300 (EI) and Jeol SX-102 (FAB) spectrometer. The characteristic IR band at 1706-1746 cm-1 (C=O) and at 1106-1146 cm-1 (C-O) confirmed the formation of acetoxylated products. The electrooxidative acetoxylation of aromatic systems were also confirmed by ¹H NMR (CDCl3) spectra and mass spectra of products by comparison with authentic samples.
The acetoxylated products viz. side chain substituted and nuclear ring substituted acetoxy aromatic systems were obtained from various aromatic substrates are shown in Figure 1 & Figure 2.
In summary, the acetoxylation of various aromatic side chain and aromatic nuclear ring was carried out with simple non-aqueous solvent and supporting electrolyte at room temperature via electro organic synthesis (EOS). The electro organic synthesis is expected to be an important organic synthetic tool in future because of its diverse application in industry in addition to compatibility with green chemistry22, 23. There is no requirement of any hazardous reagent, hence this electrochemical synthesis is simple and ecofriendly therefore it is a part of green chemistry.
Acknowledgements
Among the authors, one (Manish Kumar Srivastav) is thankful to CSIR to award senior research fellowship and R.S.I.C. Chandigarh and C.D.R.I. Lucknow for spectral analyses.
References
- Machado M. D., Perez-Pariente J., Sastre E., Cardoso D., de Guerenu A. M., Appl. Catal., A-General, 203(2), 321, (2000)
- Yadav G. D., Kulkarni H. B., React. Funct. Polym., 44(2), 153, (2000).
- Liu O. L., Chen H. F., J. Membr. Sci., 196(2), 171, (2002).
- Balakrishnan T., Rajendran V., J. Polym. Sci. Part A-Polym. Chem., 35(4), 727, (1977).
- Manohar B., Reddy V. R., Reddy B. M., Synth. Commun., 28(17), 3183, (1998).
- Ozturk G., Gumgum B., Akba O., Catal. Lett., 82(3), 233, (2002).
- Degner D., Top. Curr. Chem., 148, 1-95, (1988).
- Feroci M., Orisni M., Palombi L., Inesi A., Green Chem., 9, 323-325, (2007).
- Fry A. J., ‘Synthetic Organic Electrochemistry’, 2nd ed., Wiley Interscience Publication, 255, (1989).
- Khodaei M. M., Alizadeh A., Pakravan N., J. Org. Chem., 73(7), 2527-2532, (2008).
- Leung M., Herz J., Satzberg H. W., J. Org. Chem., 30, 310, (1965).
- Harvey D. R., Norman R. O. C., J. Chem. Soc., 4860, (1964).
- Wilson C. L., Lippicott W. T., J. Am. Chem. Soc., 78, 4290, (1956).
- Eberson L., ‘Chemistry of the Carbonyl Group’ Ed., Interscience Publishers Inc., London.
- Herk L., Feld M., Szware M., J. Am. Chem. Soc., 83, 118, (1961).
- Bookmain G., Frintz H. P., Electrochem. Acta., 21, 1099, (1976).
- Fry A. J., ‘Synthetic Organic Electrochemistry’, 2nd ed., Wiley Interscience Publication, 258, (1989).
- Perrin C. L., Progr. Phys. Org. Chem., 3, 220, (1965).
- Kumar S., Sharma L. K., Singh R. K. P., J. Indian Chem. Soc., 83, 1160, (2006).
- Singh R. K. P., Kumar A., Kumar S., Yadav K. L., J. Indian Chemical Soc., 79, 695, (2002).
- Mann C. K., Barnes K.K., ‘Electrochemical Reactions in Non-aqueous Systems’, Marcel Dekkar, Inc, New York, 6, (1970).
- Matthews M., Pure Appl. Chem., 73, 1305, (2001). 23. Steckhan E., Arns T., Hieneman G., Chemosphere, 43, 63, (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









