A Mild Synthesis, Characterization and Spectral Properties of Some New Substituted Cinnamamides and Substituted A-B Unsaturated Acids
Alok K. Pareek*, P. E. Joseph and Daya S. Seth
Department of Chemistry, School of Chemical Sciences, St. John’s College, Agra - 282 002 (India).
The present investigation deals with the evaluation of various Newly Substituted Cinnamamides bearing chloro, methoxy, methyl groups have been obtained by the condensation of substituted phenyl malon anilic acids(1a,2a) with selected different substituted aromatic aldehydes by using pyridine as a condensing agent and a-b unsaturated acids have been synthesized by the condensation reactions between the substituted malon anilic acids (1a,2a) and aromatic substituted aldehydes without any condensing agent . The structures of Newly Synthesized Novel substituted cinnamamides (3a-3f, 4a-4g) and substituted unsaturated acids (5a-5g, 6a-6g) were established on the basis of their Spectral Studies and Analytical data.
KEYWORDS:Substituted Cinnamamides; Substituted a-b Unsaturated Acids; Condensation; Spectral Evaluation
Download this article as:| Copy the following to cite this article: Pareek K, Joseph P. E, Seth D. S.A Mild Synthesis, Characterization and Spectral Properties of Some New Substituted Cinnamamides and Substituted A-B Unsaturated AcidsAlok . Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Pareek K, Joseph P. E, Seth D. S.A Mild Synthesis, Characterization and Spectral Properties of Some New Substituted Cinnamamides and Substituted A-B Unsaturated AcidsAlok . Orient J Chem 2010;26(1).Available from: http://www.orientjchem.org/?p=23531 |
Introduction
Cinnamamides are an important heterocyclic compounds have been studied extensively for the last few decades which have received considerable investigative attention with regards to their syn-thesis and their broad spectrum of biological activity. Substituted cinnamamides have been reported to possess anti-convulsant1-2, inhibitors of blood platelet aggregation3, insectisides4,melanin inhibitors5,activities . In the applications which gave reduced pigment deposit on the guinea pig skin after exposure to ultra violet radiations. α-β unsaturated acids have been evaluated as β-adrenergic blocking agents6,7. substituted unsaturated acids have also been found to possess diuretics8, anti-inflammatory and anti-arteriosclerosis remedies9 activities. Some derivatives of unsaturated acids have been reported as bronchiodilators10, fungicides11, symp-atholytics12 activities.
In this laboratory by various workers a number of substituted cinnamamides and substituted α-β unsaturated acids have been synthesized13-21.
In the present study we have synthesized a series of some new substituted cinnamamides and substituted α-β unsaturated acids. They have been synthesized by the condensation of aliphatic and aromatric aldehydes with N(R) phenyl malon anilic acids (1a,2a) with and without any condensing agent as pyridine.
Experimental
Material and Methods
All the chemicals used in the synthesis were obtained from Sigma-Aldrich Company. All the melting points were taken in open capillary tubes and were uncorrected. Thin layer chromatography (TLC) was conducted with silica-gel-coated AI plates (Merck) to check the purity of the newly synthesized compounds. The IR spectra were recorded in(Kbr-disc) method on Perkin-Elmer spectrum RX-1 FT-IR spectro-photometer at Central Drug Research Institute (CDRI) Lucknow.
The identity of newly synthesized compounds was confirmed by analytical data, elemental analysis, molecular formula, molecular weight, melting point, yield%, colour are recorded in the Table -1 and IR Spectral data are recorded in the Table -2.
General procedure of the Synthesis of N(R) phenyl malon anilic acid (1a, 2a)
To the substituted aniline (2-methoxy-5-methyl, 3-chloro-4-methoxy ; 0.025 mole ) and di-ethyl malonate (0.05 mole) was added with the catalyst DMF and then refluxed for about 45 minutes, after cooling, filter the solution and then add ethanol (20 ml) with a solution of Na2CO3 (20 ml) to it, after that the reaction mixture was hydrolysed for 30-45 minutes, filtered and concentrated HCI was added to it, the solid product was separated, filtered, washed with cold water, recrystallized from saturated solution of NaHCO3, was identified as N(R) phenyl malon anilic acid (1a, 2a) .
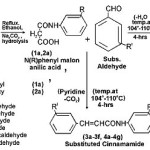 |
Scheme 1 Click here to View scheme |
General procedure of the Synthesis of Cinnamamides (3a-3f, 4a-4g)
A mixture of N(R) phenyl malon anilic acid (1a, 2a ; 0.001mole) and substituted aldehyde (0.001 mole) in equimolar quantities (1:1), with a trace of pyridine was added. The reaction mixture was heated in an oil-bath at maintained temperature 104o-110oC, brisk effervescence took place during refluxing, after cooling treated with (20 ml) solution of NaHCO3, filtered , thus the obtained residue was washed with hot water , recrystallized by aqueous ethanol, thus the analysed compound was identified to be N(R) phenyl Cinnamamide(3a-3f,4a-4g).
General procedure of the Synthesis of α-β unsaturated acid (5a-5g, 6a-6g)
A mixture of N(R) phenyl malon anilic acid (1a,2a ; 0.001 mole) and substituted aldehyde (0.001 mole) was heated in an oil-bath at 104o-110oC for 4-hours without any condensing agent, after cooling, and treated with saturated solution of NaHCO3 (20 ml), filtered, NaHCO3 extract gave a precipitate on acidification with concentrated HCI, filtered, washed with hot distilled water , recrystallized with aqueous ethanol. Thus the product obtained was identified to be benzal-N(R) phenyl malon anilic acid (5a-5g, 6a-6g).
Results and Discussion
The IR spectrum of the newly synthesized compounds of substituted cinnamamides and substituted α-β unsaturated acids have been recordedin the frequency region 4000-450 cm-1 are furnished in the Table-2. The IR (Kbr) spectrum of N(2-methoxy-5-methyl) phenyl 4-hydroxy cinnamamide 3e shows stretching vibrations at 3021.5 cm-1 indicates -NH, absorption at 1680.9 cmrepresents -CONH stretching vibrations, absorption at 1598.7 cm-1 indicates -C=C, absorption at 2362.0 cm-1 show HC=C stretching vibrations,absorption at 670.9 cm-1 represents the mono substitutions.These observations are support to the assigned structures of compounds 3e&3f, 4b&4e and other compounds 3a-3d, 4a,4c-4d, 4f-4g.The IR Spectra of 2-chlorobenzal -N-(2-methoxy-5-methyl) phenyl malon anilic acid5b shows stretching vibrations at 3021.9 cm-1 indicates -NH, stretching vibrations at 1697.9 cm-1 represent -CONH, stretching vibrations at 1598.0 cm-1 show -C=C and stretching vibrations at 2360.5 cm-1 indicates HC=C, stretching vibrations at 671.6 show mono substitution ring. All the above observations are lent support to the assigned structure of compounds 5b&5g, 6c&6d and other compounds 5a, 5c-5f, 6a-6b, 6e-6g .
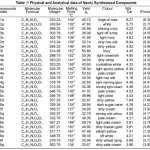 |
Table 1 Click here to View table |
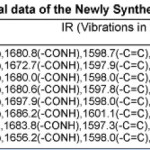 |
Table 2 Click here to View table |
The above observations are agreed with the assigned structures of all the mentioned compounds, they were found to possess higher melting points shows thermal stability and also have characteristic colouring properties.
References
- J.Lee, Yiyao Gongye,16(10): 465-7 Ch. (1985).
- S.Peng, W.Liu, J.Zhuo and Y.Pei, J.Mole.Sci. 4(2): 163-7 (1986).
- I.Katsumi, H.Kondo, K.Yamashita, T.Hidaca,K.Ho Soe, T.Yamashita and K. Watanabe, (Kanegafuchi Chem. Ind. Co.) Jpn. Kokai Tokyo Koho JP 61, 57, 567 (86,57,567) (1986).
- D.G.Kuhn(American Cyanamid Co.) U.S.us 4: 659, 857 (1987).
- K.Hori, K.Nakamura, M.Kawai, I.Mogi, G.Imokawa and N.Takoyashi(Kao Corp.) Jpn.Kokai Tokyo Koho JP 62, 56, 459(87 56,459) 12 March (1987).
- K.Nakagawa, M.Uchita and K.Oka(OtsukaPhar. Co. Ltd.) Jpn. Kokai Tokyo Koho JP 76, 56, 462, 18 May (1976).
- B.Jan, P.Stephen, M.Kelly Standifer, I.Takashi, k.Yasuhiro, W.John and J.Pitha, J.Med. Chem., 30(9), 1563-66 (1987).
- M.Uchita and K.Nakagawa,Otsuka Phar. Co.Ltd. Jpn. Kokai Tokyo Koho JP 79, 32, 485 (1979).
- T.Nishi, H.Ueda and K.Nakagawa (OtsukaPhar. Co. Ltd.),Jpn. Kokai Tokyo Koho JP 79,32, 481 (1979).
- S.Yoshizaki, S.Tamada, N.Yo and K.Nakagawa (Otsuka Phar. Co. Ltd.) Jpn.Kokai Tokyo Koho JP 78, 46, 983, 27 April (1978).
- S.Inoue, N.Yamashita and T.Uematsu (Sunutomo Chem. Co. Ltd.) Jpn. Kokai Tokyo Koho JP 78, 91, 136, 10 August (1978).
- Otsuka Phar. Co. Ltd. Jpn. Kokai Tokyo KohoJP 57, 175, 168(82,175,168) (1982).
- Pandya and Vahidy, Ibid, 2: 402 (1935).
- P.I.Ittyerah and K.C.Pandya,Proc. IndianAcad. Sci., 13(2): 119 (1941).
- M.V.George and P.I.Ittyerah, AgraUniv.,J.Res.Sci., 4(2): 551 (1955).
- O.P.Singhal, Khetan and P.I.Ittyerah, J.IndianChem.Soc., 42: 480 (1965).
- I.M.Hora, Ph.D.Thesis, Agra Univ., Agra (1969).
- R.K.Jain, D.S.Seth and B.C.Banerji,AgraUniv., J.Res.Sci., XXIX, Pt.III, 67-68 (1980).
- Arun Kumar, Ph.D. Thesis, Agra Univ., Agra (1981).
- Mukti Kalani, Ph.D. Thesis, Agra Univ., Agra (1989).
- S.Bhatnager, Ph.D. Thesis, Agra Univ., Agra (1990).

This work is licensed under a Creative Commons Attribution 4.0 International License.









