A highly selective liquid-liquid extraction technique for the extraction and separation of hafnium (IV) with hexaacetato calix(6)arene
C. M. Swamidoss1 and D. D. Malkhede2
1Department of Chemistry, Sant Gadge Baba Amravati University, Amravati (India).
2Department of Chemistry, University of Pune, Pune (India).
Solvent extraction of Hafnium(IV) was carried out using 1
KEYWORDS:Solvent extraction; Hafnium(IV); Hexaacetato Calix(6)arene
Download this article as:| Copy the following to cite this article: Swamidoss C. M, Malkhede D. D. A Highly Selective Liquid-Liquid Extraction Technique for the Extraction and Separation of Hafnium (IV) With Hexaacetato Calix(6)Arene. Orient J Chem 2010;26(1). |
| Copy the following to cite this URL: Swamidoss C. M, Malkhede D. D. A Highly Selective Liquid-Liquid Extraction Technique for the Extraction and Separation of Hafnium (IV) With Hexaacetato Calix(6)Arene. Orient J Chem 2010;26(1). Available from: http://www.orientjchem.org/?p=23483 |
Introduction
As it is well known, zirconium and Hafnium have very similar chemical properties and are commonly referred to as “chemical isotopes1. Hafnium does not occur individually but only in association with Zirconium2. Its consists mostly of 2% of the Zirconium content but in the mineral alvite, its content exceeds that of Zirconium3. It has a melting point of 2222° C with ± 30° C4. Hafnium and its other group members are quadrivalent, yet they resemble the members of Group III as the absorption of Hydrogen is concerned5. Hafnium is used as a control material in water cooled nuclear reactors and rectifiers. Hafnium is also used for alloying with Iron, Titanium, Aluminium and other metals.
Liquid-liquid extraction enjoys a favoured position among the separation techniques because of its ease, simplicity, speed and wide scope6. Katsuta et. al. worked on the solvent extraction separation of Hf(IV) with acetyl acetone7. Ramachandra Reddy et al., worked on the Solvent Extraction of Hafnium(IV) from acidic chloride solutions using Cyanex 3028, PC-88A9, and LIX 84-IC10. Tagdhizadeh et.al. worked on the stoichiometric relation for extraction of Zirconium and Hafnium from acidic nitrate solutions with Cyanex 27211,12, and the determination of optimum process conditions for the extraction and separation of Zirconium and Hafnium by Solvent extraction13. Hwa Young Lee et al., worked on the stoichiometric relation for extraction of Zirconium and Hafnium from acid chloride solution with Versatic acid 1014. Until now, no work has been done on the extraction and separation of Hafnium(IV) with Hexaacetato Calix(6)arene. The complexation phenomena have played an important role in the history of chemistry. Calixarenes are macrocyclic, oligomers that possess the capability for cationic, anionic and molecular inclusion15. Calixarenes as parent compounds are sparingly soluble and high melting crystalline solids. By functionally modifying either the upper and/or lower rim it is possible to prepare various derivatives with differing selectivities for various guest ions and small molecules. Calixarenes lend themselves well to many applications because of the multiplication of options for such structural elaboration16. Within supramolecular compounds Calix(n)arene finds several advantages over crown ethers and cryptands17. Calixarenes modified by chemical reactions may be considered as “molecular platforms” to which functional groups are fixed. There are numerous possibilities to obtain new Calixarenes. This demonstrates the wealth of class of products and the possibilities to obtain substances with interesting properties18. The advantages and novelty of the proposed method are-
The method involves a single step and is highly efficient.
There is no media required for extraction and very minimum time is required.
The method is very useful in separating Hafnium(IV) from other multicomponant mixtures.
Experimental
Apparatus and reagents
A systronics UV-Visible spectrophotometer (Model No- 108) with matched 10 mm quartz cuvettes and a digital pH meter (Systronics Model No-361) with combined glass and calomel electrodes were used.
The Hexaacetato Calix(6)arene was synthesized in our laboratory19. The purity of the ligand was checked by its melting point, elemental analysis and spectral characterization. 1 x 10-4 M solution of reagent was prepared in Xylene. A stock solution containing 100 mg/ml of Hafnium(IV) was prepared by dissolving Hafnium oxychloride in 2% HCl.
0.02% Xylenol orange was prepared by dissolving it in distilled water.
Solvent extraction procedure
An aliquot of a solution containing Hafnium(IV) (30 mg/ml) was taken. It was adjusted to pH 2 with dilute HC1 or dilute NaOH. The total volume of the solution was made up to 10 ml with distilled water. It was then transferred to a separatory funnel. After the addition of 10 ml of 1 x l0-4 M of Hexaacetato Calix(6)arene in Xylene, the solution was vigorously shaken for 10 minutes. The two phases were allowed to settle and separate. Hafnium(IV) from the organic phase was stripped with 10 ml of 5 N Nitric acid and determined spectrophotometrically at 530 nm with Xylenol orange20. The concentration of Hafnium(IV) was computed from the calibration curve.
Results and Discussion
Extraction mechanism
Acetyl derivative of Calixarene is a neutral extractant, capable of extracting uncharged metal complexes in aqueous solution under particular conditions and also charged metal ions and complexes. At the lower pH Hf(IV) forms a stable complex with Acetyl derivative of Calix(6)arene in the organic phase. From the log-log plot it is found that the slope is 2 (Figure 1). Therefore, we can conclude that the metal to ligand ratio is 1:2.
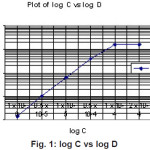 |
Figure 1: log C vs log D Click here to View figure |
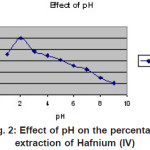 |
Figure 2: Effect of pH on the percentage extraction of Hafnium (IV) Click here to View figure |
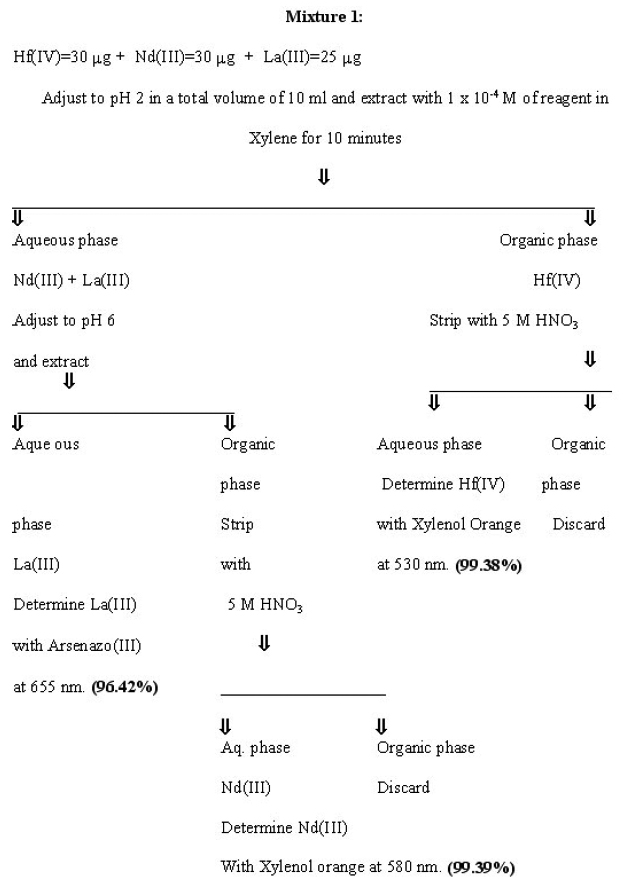
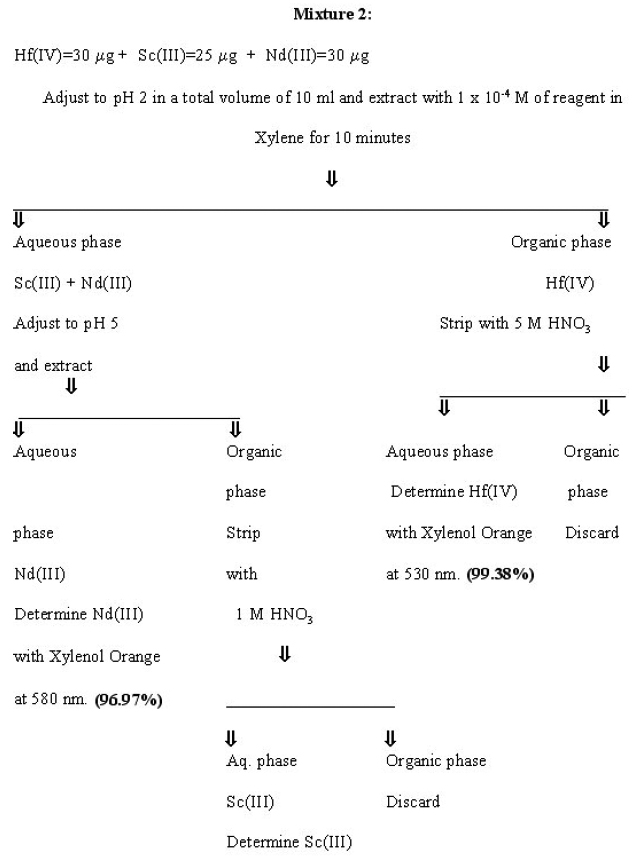
Separation of Hafnium from other multicomponent mixtures
Effect of pH and kinetics of extraction
The extraction of Hafnium(IV) was found to be quantitative at pH 2 (Figure 2). When Hafnium(1V) was equilibrated with 1 x 10-4 M of Hexaacetato Calix(6)arene in Xylene, it was observed that extraction was quantitative for 10 minutes of shaking and independent of time after 10 minutes Therefore, equilibration time was considered as 10 minutes for further investigations. Loading capacity and stripping studies:
Hafnium(IV) could be quantitatively extracted and stripped up to 50 µg/ml using lx 10-4 M of Hexaacetato calix(6)arene in Xylene. On increasing the concentration, extraction was not quantitative. Hafnium(IV) was stripped with varying concentrations of mineral acids as the stripping agents. With 7 N Hydrochloric acid, 5 N Nitric acid and 3 N sulphuric acid, extraction was quantitative. Therefore, 5 N nitric acid was preferred as the stripping agent because of the ease in stripping.
Effect of diluents and reagent concentration
Various solvents with varying dielectric constants were used as diluents. The extraction with Toluene, Cyclohexane, and Kerosene was greater than 95%. However, extraction was quantitative with Xylene. We preferred Xylene as the diluent because of its relatively less toxicity; it gave better phase separation and greater accuracy in results. It was observed that solvents having low dielectric constants show extraction of Hf(IV) above 90%. Hafnium(IV) was extracted with 10 ml of reagent concentration varying from 10-6 M to 2 × 10-4 M. The extraction was quantitative with 1 × 10-4 M of reagent. It was not worth using high reagent concentrations exceeding 1 × 10-4 M since it did not materially improve the extraction.
Table 1: Effect of Foreign Ions on the extractive determination of Hf(IV) = 30 mg
| Foreign Ion | Added As | Amount tolerated (mg) | Foreign Ion | Added As | Amount tolerated (mg) |
| Li+ | LiCl | 600 | Fe2+ | FeCl | Interfering |
| Na+ | NaCl | 600 | Fe3+ | FeCl | Interfering |
| K+ | KCl | 600 | Sc3+ | Sc O | Interfering |
| Mg2+ | MgCl2 | 600 | Th4+ | Th(NO | 30 |
| Co2+ | CoCl | 600 | Bi3+ | Bi(NO ) | 30 |
| Zn2+ | ZnCl | 600 | Pb2+ | Pb(NO ) | 600 |
| Mn2+ | MnCl | 600 | Sm3+ | Sm(NO ) | Interfering |
| Cd2+ | CdCl | 600 | Cl– | NaCl | 600 |
| Hg2+ | HgCl | 600 | F– | NaF | 30 |
| Sn2+ | SnCl | 600 | Br– | NaBr | 600 |
| Ni2+ | NiCl | 600 | I– | NaI | 600 |
| Sr2+ | SrCl | 300 | CO32- | Na2CO | 600 |
| Ba2+ | BaCl2 | 300 | SO32- | Na2SO | 600 |
| V5+ | V2O5 | 600 | SO42- | Na2SO | 450 |
| Mo6+ | MoO3 | 600 | PO 3- | Na3PO3 | 600 |
| Cu2+ | CuCl2 | 600 | NO – | NaNO2 | 150 |
| Zr4+ | ZrOCl2 | Interfering | NO – | NaNO3 | 150 |
| Ca2+ | CaCl2 | 600 | Oxalate | Na6C6O4 | 600 |
| Cr3+ | CrCl3 | 600 | tartarate | Na2C4H4O6 | 600 |
| Al3+ | AlCl3 | 600 | EDTA | C10H14O8Na2N2 . 2H2O | 600 |
Temperature Effect
The effect of temperature was studied on the extraction of Hafnium(IV) from 283 K to 353 K. It was found that the percentage of extraction increased from 283 K to 303 K from 96.25% to 99.38% and thereafter remained constant until 353 K.
Effect of Foreign Ions
The extraction of Hafnium(IV) was carried out in the presence of other foreign ions. Most of the I group, II group and transition metal ions were found to have a tolerance limit of 600 ppm. Interfering ions were Fe2+, Fe3+, Zr4+, and Sc3+ (Table-1).
Conclusion
The highlights of the proposed method are as follows:
The method is simple, quantitative and requires a single step.
The reagent can be synthesized easily in the laboratory at low costs.
There is no emulsion formation.
Pre-equilibrium and multiple extraction of the organic phase is not required.
The extraction of Hafnium(IV) was found to be quantitative at room temperature.
The method can be extended for the determination of Hafnium in real samples and synthetic mixtures also.
The method is also eco-friendly.
Acknowledgements
The authors wish to thank the University Grants Commission for the financial support.
References
- Lee, J. D., Concise Inorganic Chemistry, Fifth edition, Chapman and Hall, London, (1996).
- Holleman, A. F. and Wiberg, E., Inorganic Chemistry, Academic Press, London, 1316 (2001).
- Greenwood, N. N. and Earnshaw, A., Chemistry of the Elements, Pergamon Press, 1112 (1989).
- Cotton, F. A., Wilkinson, G., Murillo, C. A., Bochmann, M., Advanced Inorganic Chemistry, 6th edition, Wiley and Sons, 880 (2003).
- Sathyaprakash, Advanced Chemistry of rare elements, 4th edition, S. Chand, 489 (1986).
- Morrison, G. H. and Freiser, H., Solvent Extraction in Analytical Chemistry, Wiley and Sons, New York, 3 (1966).
- Katsuta, S., Yanagihara, H., Solvent Extraction and Ion Exchange, 15: 577 (1997).
- Ramachandra Reddy, B., Rajesh Kumar, J., Phani Raja, K., Varada Reddy, A., Minerals Engineering, 17: 939 (2004).
- Reddy, B. R., Kumar, J. R., Reddy, A. V., Minerals Engineering, 17: 553 (2004).
- Ramachandra Reddy, B. and Rajesh Kumar, J., Separation and Purification Technology, 42: 169 (2005).
- Tagdhizadeh, M., Ghasemzadeh, R., Ashrafizadeh, S. N., Ghannadi, M., Hydrometallurgy, 96: 77 (2009).
- Tagdhizadeh, M., Ghasemzadeh, R., Ashrafizadeh, S. N., Saberyan, K., Maragheh, M. G., Minerals Engineering, 20: 1401 (2007).
- Tagdhizadeh, M., Ghasemzadeh, R., Ashrafizadeh, S. N., Saberyan, K., Maragheh, M. G., Hydrometallurgy, 90: 115 (2008).
- Lee, H. Y., Kim, S. G., Oh, J. K., Hydro-metallurgy, 73: 91 (2004).
- Gutse, C. D., Calixarenes, The Royal Society of Chemistry, (1989).
- McMahon, G., O’Malley, S., Nolan, K., Diamond, D., Important Calixarene Derivatives- their synthesis and applications, ARKIVOC 23 (2003).
- Khopkar, S. M., Analytical Chemistry of Macrocyclic and Supramolecular compounds, Narosa Publishing House, (2002).
- Pemn, R., Lamartine et Monique Pemn, R., Pure and applied Chem, 65: 1549 (1993).
- Malkhede, D. D., Dhadke, P. M., Khopkar, S. M., Analytical Sciences, 15: 789 (1999).
- Snell, F. D., Photometric and Fluorometric methods of Analysis, Wiley publication, New York, (1978).

This work is licensed under a Creative Commons Attribution 4.0 International License.









