Theoretical Study of The Heterocyclic Molecules Reactivity in The Normal Electron Demand Diels-Alder Reaction
Sameh ayadi, Khaled Essalah, Slaheddine Krichane and Manef Abderrabba
Unite de recherche Physico-Chimie Moleculaire. IPEST, Boite postale 51, 2070 la Marsa, Tunisie.
Corresponding Author E-mail : sameh_ayadi2003@yahoo.fr
The purpose of this work is a theoretical study of the Diels-Alder reactions between, a diene 2 like 2,3-dimethylbutadiene 2a1 and 2-trimethylsilyloxybuta-1,3-diene 2b1 with a series of 6-nitrobenzofuroxane 5a-c . From a thermodynamic and orbital point view, we discuss the possibility and the stereoselectivity of these kinds reactions.
KEYWORDS:normal electron demand Diels-Alder (NEDDA); DFT method; DNBF
Download this article as:| Copy the following to cite this article: Ayadi S, Essalah K, Krichane S, Abderrabba M. Theoretical Study of The Heterocyclic Molecules Reactivity in The Normal Electron Demand Diels-Alder Reaction. Orient J Chem 2008;24(1). |
| Copy the following to cite this URL: Ayadi S, Essalah K, Krichane S, Abderrabba M. Theoretical Study of The Heterocyclic Molecules Reactivity in The Normal Electron Demand Diels-Alder Reaction. Orient J Chem 2008;24(1). Available from: http://www.orientjchem.org/?p=23026 |
Introduction
Experimental study done by auteurs shows that the addition of 2,3-dimethylbutadiene to a solution of 4-aza-6-nitrobenzofuroxan 1 in CDCl3 gives the product 3a1 and 3’a1.The auteurs carry out another experience, under similar conditions, but with a longer reaction time this reaction did not allow the isolation of 3a1 and 3’a1, but in adding advantage water in the solution, he obtained the hydrate 4, likes pale yellow solid, resulting from the reaction of 3a1. The structure of 4 was determined by X-ray crystallography, which confirm that the addition of H2O has taken place at the C10=N9 double bond [1].
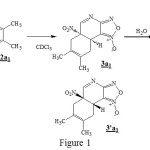 |
Figure 1 |
Recently, it has been showed that the nitrobenzofuroxan (DNBF) are able to react as a dienophile or heterodiene in the Diels-Alder reactions [2-5]. In first stage, we have choice the 6-nitrobenzofuroxane molecules which looks like the 4-aza-6-nitrobenzofuroxan 1. We have only substitue the nitrogen atome number 4 of the molecule 1 by the C-R3 groupment, then we obtient the molecules 5a-c (figure 2) which seems particulary interessants for experimental study. The diene substitution was studied by substitution of 2,3-dimethylbutadiene 2a1 by the 2-trimethylsilyloxybuta-1,3-diene 2b1. In order to find the better calculation method which lead to better convergence for these kind of system, we have optimazed molecule 4 by two different method SCF/6-31G and DFT/B3LYP with the basis set 3-21G. In second stage, we have studied the addition of diene 2a1-b1 on the 6-nitrobenzofuroxane 5a-c. In the end, from thermodynamic and orbital point of view we have discussed the possibility and the stereoselectivity of normal electron demand Diels-Alder reaction.
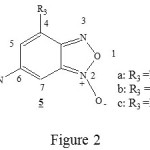 |
Figure 2 Click here to View Figure |
Theoretical Study of the Compound 4
Calculation Method
Calculations are done at SCF level with standard basis sets 6-31G of GAUSSIAN 98 program[6] and while using the DFT method of the same program with The B3LYP hybrid functional [7, 8] and basis sets 3-21G. The choice of this last method is justified by its efficiency in the treatment of system type [9]. The geometry of the various critical points on the potential energy surface was fully optimized with the gradient method available in GAUSSIAN 98. Calculations of harmonic vibrational frequencies were carry out to determine the nature of each critical point.
Results
In order to determine the reliability calculation methods in the treatment of this system type, we optimized the geometry of the compound 4. Putting in mind the stereochemistry of this molecule 4, we compare our theoretical results with the experimental data of the X-ray [1]. Table 1 regroups some geometric parameters of the optimized molecule and the X-ray data.The figure 2 shows the stereochemistry of the molecule 4 optimised at DFT level.
Table 1 : Selected optimized geometrical parameters for the compound 4.
|
SCF/6-31G |
DFT/3-21G |
X-ray [6] |
|
| N4-C5 | 1.457 | 1.455 | 1.445 |
| C7-C10 | 1.525 | 1.526 | 1.537 |
| C11-C12 | 1.348 | 1.347 | 1.327 |
| N2-C6 | 1.565 | 1.563 | 1.536 |
| C5-O5 | 1.414 | 1.412 | 1.408 |
| N4-C9 | 1.354 | 1.391 | 1.366 |
| C13-C12 | 1.487 | 1.489 | 1.499 |
| C6-C7 | 1.473 | 1.474 | 1.543 |
| C5-C6 | 1.449 | 1.448 | 1.547 |
| N4-H4 | 1.001 | 1.001 | 0.86 |
| C9-N4-C5 | 115.373 | 115.377 | 115.8 |
| N4-C5-C6 | 124.34 | 124.257 | 109.37 |
| C5-C6-C7 | 119.587 | 119.585 | 111.81 |
| C6-C7-C8 | 109.287 | 109.285 | 105.06 |
| C11-C12-C13 | 121.03 | 121.05 | 122.55 |
| C10-C11-C12 | 118.347 | 118.415 | 123.16 |
| C7-C10-C11 | 108.642 | 108.768 | 116.39 |
| C7-C8-C9 | 125.381 | 125.546 | 125.72 |
Distances are in Angstrom (A°) and Angles in degrees (°)
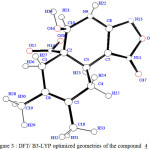 |
Figure 3 : DFT/ B3-LYP optimized geometries of the compound 4 Click here to View Figure |
The SCF/6-31G and DFT method give result which agree with the experimental X-ray data of the compound 4. We noticed that the DFT method gives the best results. Therefore the calculation method of DFT/B3-LYP with the standard basis set 3-21G is more reliable with this system type [9]. The geometry parameters data assigned to 4 is in complet agreement with our theoretical results. The distance ( d H7-H10 =2.47 A°) calculated by DFT/B3Lyp with the standard basis set 6-31G was in good accord with the distance obtained by X-Ray crystallography : d H7-H10 = 2.186 A°.
Vibrational Analysis
IR spectra calculate by DFT/3-21G method are also in accord with the experimental one (nC6=C5(theo) =1755 cm-1 et nC6=C5(exp) =1609 cm-1). We noticed that the DFT method using the functional B3LYP and the standard basis set 3-21G gives the most resultats with this type of heterocyclic molecules. Therefore, we continuous the thermodynamic study on Diels-Alder reaction between the heterocyclic molecules such as 4-aza-6-nitrobenzofuroxan 1 and 6-nitrobenzofuroxan 5a-c with the diene 2a1-b1, by using the DFT method with the functional B3LYP and the standard basis set 3-21G of the GAUSSIAN 98 program [ 6 ].
DFT Study of Diels-Alder Reaction
Like the figure 4 shows, we have studied the possibility and also the stereoselectivity of normal electron demand Diels-Alder reaction between heterocyclic molecules such as 4-aza-6-nitrobenzofuroxan 1 and 6-nitrobenzofuroxan 5a-c with the diene 2a1-b1. The diene is activated by the substituant groupement – ( CH3) or – (OSi (CH3)3. In the case of diene 2a1-b1 addition on the 6-nitrobenzofuroxane 5a-c, we have focussed our attention to study the R3 groupement on the Diels-Alder reaction. This study seems to be interessant from synthetic point of view.
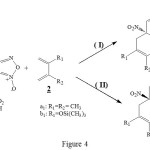 |
Figure 4 Click here to View Figure |
The orbitals Border Study
We have determinated the LUMO and the HOMO of diene 2a1-b1, by DFT/B3LYP with standard basis set method, and as well the LUMO and the HOMO of molecules 1 and 5a-c see table I. In each case of these reactions, the orbital diagramm between the molecules 1 and the diene 2a1-b1, give a very good quantitatively indication about the reactivity of our system. The most predominant interaction existe between the LUMO of dienophile and the HOMO of diene 2a1-b1. Therefore these reactions could be considered like the Diels-Alder reaction with normal electron demand [10, 11]. As example, we presente figure 5
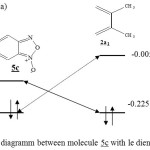 |
Figure 5 : orbital diagramm between molecule 5c with le diene 2a1. DFT/3-21G Click here to View Figure |
The table I shows, that the difference of energy HOMO-LUMO is smaller in the case of the diene 2b1 reaction with the molecule 5a that in the case of the reaction between 2b1 with 5c. Therefore the reaction should be easier in the case of the diene 2b1 addition on the molecule 5a.
Table I: The LUMO and the HOMO of diene 2a1-b1 and heterocyclic molecules such as 4-aza-6-nitrobenzofuroxan 1 and 6-nitrobenzofuroxan 5a-c
|
LUMO |
HOMO |
|
|
1 5a 5b 5c |
-0.132 -0.179 -0.149 -0.151 |
-0.254 -0.289 -0.256 -0.264 |
|
2a1 2b1 |
-0.005 -0.021 |
-0.225 -0.217 |
Study of Regioselectivity Reaction
The study of (2pz) coefficients of the border orbitals by DFT/3-21G level for the 4-aza-6-nitrobenzofuroxan 1 and 6-nitrobenzofuroxan 5a-c show that the LUMO coefficient of carbone C5 is always less then carbone C7 (Table II).This means that the addition of diene 2a1-b1, appear more reactif on the molecules 1 and the 5a-c is obtained firstly on carbone C7. This constatation is agree with experimental resultats in the case of diene 2a1 add on the 4-aza-6-nitrobenzofuroxan 1 [1 ].
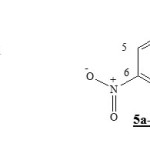 |
Scheme 1 Click here to View Scheme |
Table II : The LUMO Coefficient of carbone C5, C6 and C7of molecule 1 and 5a-c, The HOMO coefficient of carbone C’1 and C’4 of diene 2a1-b1.
|
LUMO coefficient of C7 |
LUMO coefficient of C6 |
LUMO coefficient of C5 |
|
|
1 |
0.241 |
0.255 |
-0.145 |
|
5a 5b 5c |
0.201 0.183 0.153 |
0.241 0.226 0.186 |
-0.145 -0.134 -0.138 |
|
|
HOMO coefficient of C’1 |
HOMO coefficient of C’4 |
|
|
2a 2b |
0.285 0.236 |
0.285 0.264 |
In purpose to determine the stereoselectivity of the reaction between the molecules 5a-c and the diene 2b1, we have calculated the (2pz) coefficient of LUMO for diene 2b1 and also the 2pz coefficient of LUMO which correspond to carbone C7 and C6 of molecules 5a-c and this leads to the formation of product 6. The formation of (1’)-(7) and (4’)-(6) liaisons are more favorised. See the example on Figure 5
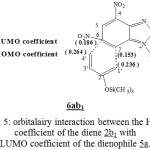 |
Figure 5: orbitalairy interaction between the HOMO coefficient of the diene 2b1 with LUMO coefficient of the dienophile 5a. Click here to View Figure |
Thermodynamic Approach
As it is shows in figure 6, we studied the interaction of this heterocyclic molecules1 and 5a-c with the diene 2a1-b1. The heterocyclic molecules react as dienophile and the molecules of 2,3-dimethylbutadiene 2a1 and 2-trimethylsilyloxybuta-1,3-diene 2b1 react as a diene, Therefore we have a normal electron demand Diels-Alder (NEDDA). We found, it is interesting to examine from a thermodynamic approach the possibility and the stereoselectivity of Diels-Alder reactions. In each case of these reactions, we calculated by the DFT / B3LYP with the standard basis set 3-21G, the variation of free energy ΔrG (Kcal.mol-1) correspond to every type of reaction.The table III regroups the different values obtained by the DFT method.
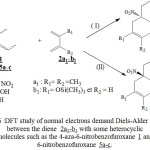 |
Figure 6 : DFT study of normal electrons demand Diels-Alder reaction between the diene 2a1-b1 with some heterocyclic molecules such as the 4-aza-6-nitrobenzofuroxane 1 and 6-nitrobenzofuroxane 5a-c.
|
The reactions (I) and (II) between the 2,3-dimethylbutadiene 2a1 with the benzofuroxane molecules 5a-c and molecule 1 are possible, since the variation of free energy are negative. On the other hand , we sudy the add of the 2-trimethylsilyloxybuta-1,3-diene 2b1 to the molecules 5a-c and 1. We notice that reactions (I) and (II) are possible and favored thermodynamically.
We also notice that the variation of free energy corresponding to reaction (I ) are more important in absolute value, than the variation of free energy relative to reaction (II). This is confirm the possibility of these reactions. Therefore the reactions (I ) give always the main product.
Table III: Thermodynamic results for some reaction calculated at the DFT/B3LYP level of theory. (Kcal.mol –1)
|
Product of reaction |
Dr E |
Dr H |
Dr G |
|
|
REaction I |
3a1 3b1 6aa1 6ab1 6ba1 6bb1 6ca1 6cb1 |
-23.4 -26.7 -17.4 -29.4 -18.9 -16.3 -12.5 -13.1 |
19.3 23.4 27.2 31.4 21.5 22.3 18.9 14.7 |
-15.6 -17.1 -20.1 -25.3 -15.6 -17.3 -10.4 -12.3 |
|
Réaction II |
3’a1 3’b1 6’aa1 6’ab1 6’ba1 6’bb1 6’ca1 6’cb1 |
-13.9 -21.1 -15.3 -20.2 -13.7 -14.8 -9.7 -11.5 |
16.3 15.2 21.3 25.7 17.3 10.4 12.3 15.2 |
-10.4 -12.3 -19.5 -23.2 -12.4 -13.6 -8.4 -10.1 |
From these results, we can give answer about some experimently question [ 1]. In fact, the addition of H2O molecule could be done only on the majority of the reaction product. For this reason, the addition of H2O is done only on the C10=N9 bond of product 3a1 which could be considered from our calculation as the majority reaction product.
In each case of reactions (I ) and (II ), we have determine the energy which leads to the formation of reaction product. We have make the follow constatation that the energy formation of endo products 6 is more weak then the energy formation of exo products 6’. This is a new confirmation of the stability of endo products which is considered as major product part of Diels-Alder reaction between the molecules 1 and 5a-c with the diene 2a1-b1.
Conclusion
We study the Diels-Alder reaction between a diene 2 like 2,3-dimethylbutadiene and 2-trimethylsilyloxybuta-1,3-diene with 6-nitrobenzofuroxane 5a-c, which is specially interesting for scientist in experimental workshop. Therefore the diene is actived by differents substituants and the 6-nitrobenzofuroxane 5 substituate by –NO2, -OH and -H. We also study the influence by R1 and R2 in the Diels-Alder reactions.Our theoretical results show, that the reactions (I) give always the main product which is the endo products.the reaction between the diene 2-trimethylsilyloxybuta-1,3-diene 2b1 and DNBF 5a gives most results than DNBF 5a with the diene 2,3-dimethylbutadiene 2a1.
References
- F. Terrier, M. Sebban, R. Goumont, J.C. Hallé, G. Moutiers, E. Buncel, J. Org. Chem. 2000, 65, 7391.
- J. C. Hallé, D. Vichard, M. J. Pouet, F. Terrier J. Org. Chem. 1997, 62, 7178.
- P. Sepulcri, J. C. Hallé, R. Goumont, D. Riou, F. Terrier, J. Org. Chem. 1999, 64, 9254.
- P. Sepulcri, R. Goumont, J. C. Hallé, D. Riou, F. Terrier, J. Chem. Soc., Perkin Trans. 2, 2000, 51.
- D. Vichard, L. J. Aley et F. Terrier, Tetrahedron Letters, 2001, 42, 7571-7574.
- M.J.Frisch, G.W. Trucks, H.B.Schlegel, G.E. Scuseria, M.A.Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A.Montgomery, Jr., R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D.Daniels, K.N. Kudin, O. Farkas, J. Tomasi, V.Barone, M.Cossi, R. Cammi, B. Mennucci, C.Pomelli, C. Adamo, S. Clifford, J. Ochterski, G.A. Petersson, P.Y. Ayala, Q. Cui, K. Morokuma, P.Salvador, J.J.Dannenberg, D.K.Malick, A.D. Rabuck, K.Raghavachari, J.B.Foresman, J.Cioslowski, J.V.Ortiz, A.G. Baboul, B.B.Stefanov, G.Liu, A.Liashenko, P. Piskorz, I.Komaromi, R. Gomperts, R.L.Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W.Gill, B. Johnson, W.Chen, M.W. Wong, J.L.Andres, C.Gonzalez, M. Head-Gordon, E.S.Replogle, J.A.Pople, Gaussian 98, Revision A.11, Gaussian, Inc., Pittsburgh PA, 2001.
- Lee C.; Yang W.; Parr R.G. Phys. Rev. B, 1988, 37, 785.
- Becke A.D Phys. Rev. A.; 1988, 38, 3098.
- Goldstein E.; Brett Beno; Houk K. N.; J. Am. Chem. Soc. 1996, 118, 6036.
- Carruthers W.Cycloadditions Reactions in Organic Synthesis ; Tetrahedron Organic Chemistry Series, Vol 8;Pergamon; New York, 1990.
- Fleming I, Frontier Orbitals and Organic Chemical Reactions, John Wiley and Sons, New York, 1976. b) Houk K.N.; Li Y.; Evanseck J.D. Angew. Chem, Int. Ed. Engl. 1992, 31, 682.

This work is licensed under a Creative Commons Attribution 4.0 International License.









