Colour Reactions of Chalcones and Their Mechanism (A Review)
Bhavana Sharma1*, S. C. Agrawal2 and K. C. Gupta3
1Ms Bhavana Sharma, 1/45 Kirti Nagar, Sadar Bhatti Road, Agra - 282 010 India.
2Department of Chemistry, Agra College, Agra - 282 010 India.
3Anand College of Pharmacy, Keetham, Agra - 282 007 India.
Corresponding Author E-mail: bhavana.chem@gmail.comChalcones are open analogues of flavonoids and give bright red to purple colours with different reagents which can be used to distinguish them from other flavonoids such as flavanones, flavones, aurones etc. Studies show that the bathochromic shift arises due to the formation of carbonium ion at the carbonyl group (>C = O) of the chromogen (ArCOCH = CHAr) as an intermediate which produces deeper colour during the reaction.
KEYWORDS:Chalcone; halochromy; benzylideneacetophenone
Download this article as:| Copy the following to cite this article: Sharma B, Agrawal S. C, Gupta K. C. Colour Reactions of Chalcones and Their Mechanism (A Review). Orient J Chem 2008;24(1). |
| Copy the following to cite this URL: Sharma B, Agrawal S. C, Gupta K. C. Colour Reactions of Chalcones and Their Mechanism (A Review). Orient J Chem 2008;24(1). Available from: http://www.orientjchem.org/?p=23307 |
Introduction
Benzylideneacetophenones constitute a class of naturally occurring pigments, which are often referred to as “Chalcones’’ (Figure 1). The term was first coined by Kostanecki1 who did pioneering work in the synthesis of natural coloring compounds.
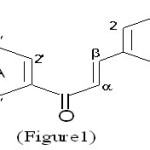 |
Figure 1 Click here to View Figure |
The synthesis of chalcone, the parent member of the series, has been accomplished in a variety of ways, but perhaps the simplest method is the one involving the Claisen-Schmidt reaction. This is the reaction of acetophenone with benzaldehyde in the presence of aqueous alkali or Sodium ethylate, resulting in the formation of a, b -unsaturated ketone2.
C6H5COCH3 + C6H5CHO → C6H5COCH = CHC6H5 + H2O
Chalcones undergo a colour change to a deeper shade (by deeper shade is meant a displacement of absorption maxima towards longer wavelength), when treated with different reagents. This phenomenon is known as ‘‘halochromy’’3.
The present review is intended to present some observations on the colour reactions given by chalcones with different reagents which can be used to distinguish them from other flavonoids.
Chemistry of the Colour Tests for Chalcones with Different Reagents
The commonly used reagents for colour tests of chalcones are (1) Alcoholic Ferric Chloride, (2) Concentrated Sulfuric acid, (3) Sulfuric acid-Nitric acid, (4) Sulfuric acid-Acetic anhydride, (5) Sodium Borohydride and HCl acid, (6) Wilson’s boric test and (7) Antimony pentachloride.
Alcoholic Ferric Chloride Test
This test is applicable to only hydroxy chalcones and is fact the characteristic of phenolic group and is due to the formation of complex iron salt4. The nature of the colour developed have however a definite relation with the orientation of the phenolic –OH in the chalcone molecule.
When a small amount (nearly 50 mg) of the chalcone was dissolved in 3ml of ethanol and then two drops of freshly prepared alcoholic FeCl3 solution was added to it. Instantaneously definite shades of colours (i.e. blue, wine red, blue black, violet or green colours) were produced.
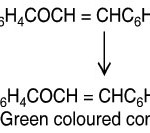 |
Scheme 1 Click here to View Scheme |
It is worth while to mention that in certain cases the colours produced were not permanent but transient so careful observation is descramble in performing this test.
Concentrated Sulfuric acid5
Chalcones exhibit beautiful halochromic effect when wetted with conc. H2SO4. The colours imparted are usually transient. When chalcone dissolved in conc. H2SO4 the coloured carbonium ion produced. The resonance must of course be regarded as extended to the benzene ring.
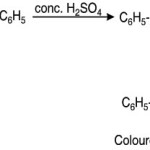 |
Scheme 2 Click here to View Scheme |
Sulfuric acid–Nitric acid6
When the intensely coloured solution of chalcone in conc. H2SO4 acid is treated with a little conc. HNO3 acid, characteristic colour changes occur. This change involves nitration of chalcones rather than oxidation, and the resulting nitrochalcone exhibits weaker halochromy, as compare to unsubstituted/substituted chalcones.
This test involves the formation of nitronium ion which attack on the 3′-position of chalcone molecule and give nitrochalcone which exhibit weaker halochromy as compare to parent chalcone.
HNO3 + H2SO4 → NO+2+H3O+2HSO–4
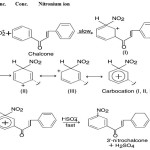 |
Scheme 3 Click here to View Scheme |
The nitration of chalcone takes place at 3′-position which is supported by the nitration of chalcone with small amount (1mole) of copper nitrate-acetic anhydride7 as well as with cerric ammonium nitrate in chloroform8.
Flavanones and flavones do not under go nitration with H2SO4–HNO3 acid so this can be used to distinguish chalcones from flavanones and flavones. The chalcone gives orange to yellow colour while methoxy chalcone gives reddish orange to yellow colour with this reagent.
Sulfuric Acid–Acetic anhydride
King and White9
found that substituted (hydroxy, methoxy) chalcones in acetic acid solution produce deep colour (orange to purple) when treated with a drop or two of conc. H2SO4 acid. The test is negative with other flavonoids except flavanones and aurones. The bathochromic shift arises due to the addition of acetic anhydride to the chalcone (in conc. H2SO4) has been rationalized in terms of stability conferred on the carbonium ion (III ® IV) by acetylation with acetic anhydride.
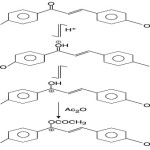 |
Scheme 4 |
On treatment with an Ac2O solution (0·2% W/V) containing two drops of conc. H2SO4 at 10–15ºC. Chalcones range from orange to purple colours which are listed in table 1.
Krishnamurthy10
et al. observed that the positive response given by flavanones is due to the formation of traces of chalcones formed during the reaction as a resulting of ring opening and their immediate conversion into coloured product. They also introduced new reagents like HClO4 and ZnCl2 (Lewis acid) which are capable of generating acetylium ions from Ac2O serve equally well in the place of H2SO4 acid.
Table 1: Colour obtained with Conc. H2SO4-Ac2O Solution
| Chalcone | Colour |
|
Chalcone (unsubstituted) 4′-Methoxy 4-Methoxy 4, 4’–Dimethoxy 3, 4, 4’–Trihydroxy 3, 4’–Dihydroxy–4–methoxy 2’–Hydroxy–3, 4, 4’–trimethoxy 2’–Hydroxy–3, 4, 4’–trimethoxy 4–Dimethylamino 3, 4, 4’–Tribenzyloxy |
YellowOrangeRedPurpleOrangeCerise
Blue change into Red Deep pink Wine red Red |
Sodium Borohydride and HCl Acid
Transient colours are developed when chalcones after reduction with sodium boro hydride, are treated with conc. HCl acid. This colour test can therefore be utilized for their identification. The mechanism of this colour reaction can be illustrated with reference to 2’–hydroxy–3, 4 4′, 6,–tetramethoxychalcone (I).
Krishnamurthy and Seshadri10
noted that when chalcone (I) treated with sodium borohydride—HCl acid reagent it gave purple colour. This observation provided evidence to demonstrate that carbonium ion (III) arising in accordance with Geissman’s scheme is involved in the colour test.
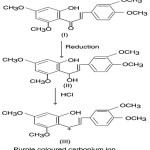 |
Scheme 5 |
More satisfying evidence in support of the above scheme was provided by the following experiment. Selective reduction of 4–methyoxy- chalcone (IV) with sodium borohydride gave a colourless unsaturated alcohol (V) (m.p. 48º). Its IR, spectrum showed the presence of hydroxyl and no carbonyl. It was different from the propanol (VI). It was different from the propanol (VI) prepared from 4-methoxy chalcone (IV) by Pt/H2 reduction and it also gave the propanol (VI) by further catalytic reduction. It was very sensitive to acid and gave an immediate red colour with ethanolic HCl while the propanol (VI) showed no change.
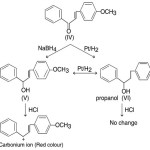 |
Scheme 6
|
Wilson’s Boric Test
Chalcones having an ortho-hydroxy or methoxy group give a positive colour reaction with borocitrci acid reagent11 as for example, 2′, 4, 4′, 6′ – tetrahydroxychalcone. The reagent is prepared by mixing equal parts of two separate solution, one composed of 100 c.c. of absolute acetone saturated with boric acid, and the other 100 c.c. of absolute acetone containing 10 gm of anhydrous citric acid (made by allowing crystalline acid to efflorescence completely in air at 30–40º, then heating it in a thin layer to 100º for 2 hours. For determining the reactivity, approximately 5 mg of chalcone derivative is dissolved in about 1.c.c. of dry acetone and the solution divided into two equal portions. To one portion is added 2c.c. of boric acid-citric acid-acetone reagent, and the other portion is diluted to an equal volume with a mixture of equal parts of the citric-acetone solution. The colours of the two tubes are compared at the end of a few minutes and any definitely stronger colour in the boric acid containing tube is regarded as a positive reaction.
This test is also very specific for partially methylated hesperatin12, 5–hydroxy and methoxy chalcone13.
Antimony pentachloride
Various chalcones treated with SbCl5 in CCl4 yields intense red or violet precipitates, which are characteristically different from the yellow or orange precipitates from flavones, flavanones and flavonols.
In this test 5–10 mg of the compound is dissolved in 5 c.c of anhydrous CCl4 and 1 c.c. of 2% anhydrous CCl4 solution of SbCl5 is added.
Mechanism
The results indicate that the formation of chalcone complex, which, as, with the complexes from stannic chloride14 is characterised by strong halochromism, depends upon the >C = O group activated by the double bond, and the colours, which differ only within a limited range of wavelengths in accordance with the number of substituents15 indicate the presence of the –COCH = CH– group united to two benzene rings.
The SbCl5 test makes it possible to distinguish chalcone from flavonic pigments and to ascertain the purity of flavones, flavonols and flavanones synthesized from the respective hydroxy chalcones, which remain as impurities difficult to eliminate even by repeated crystallisation. The SbCl5 reaction is extremely sensitive16 e.g. a positive test for 2-hydroxy-3-, 4, 4′- trimethoxychalcone is obtained at a concentration of 1 in 1000000 (1 PPM). A positive test is obtained with the chalcones listed in table 2.
Table 2: Colours obtained with various Chalcones in the SbCl5 test
| Chalcone | Colour |
| 2-Hydroxy-2-methoxy2-Hydroxy-4, 4′, 5-trimethoxy2-Hydroxy-4,5-dimethoxy-3′,4′-methylenedioxy2-Hydroxy-3, 4, 4′, 5-tetramethoxy2, 2′-Dihydroxy-3′,4′, 5, 6, 6′-pentamethoxy4-Methoxy
3′, 4′-Dimethoxy 3′, 4′-Methylenedioxy 2′, 4, 5-Trimethoxy 2, 3, 4, 4′, 5-Pentamethoxy 2, 3, 4-Trimethoxy-3′, 4′-methylenedioxy |
Blood redBlood redCherry redBrick redViolet redRed
Cherry red Violet red Dark red Red Cherry red |
In present work we have also studied the halochromy of some new chalcones synthesized by Claisen-Schmidth condensation of substituted acetophenone and substituted benzaldehydes. These chalcones gave deep colours (due the bathochromic shift in lmax ranges from +12 to +90) with alcoholic FeCl3, conc. H2SO4 and conc. Ac2O–H2SO4 listed in table 3.
Table 3: Colours given by Various Chalcones with Different Reagents
| Chalcone | MeOH | Alc. FeCl3 | Conc. H2SO4 | Ac2O–H2SO4 |
| 4, 4′-Dihydroxy4, 4′-Dihydroxy-3-methoxy2-Chloro-4′-hydroxy4-Hydroxy-4′-nitro
4-Hydroxy-3-methoxy-4′-nitro 4-Dimethylamino-4′-nitro 4′-Amino-4-hydroxy 4′-Amino-4-hydroxy-3-methoxy 4′-Amino-4-dimethylamine 4′-Amino-2-chloro 4-Hydroxy-3′-nitro 4-Hydroxy-3-methoxy-3′-nitro 4-Dimethylamino-3′-nitro
|
YellowYellowColourlessYellow
Yellow Yellow Orange Yellow Orange Pale yellow Brown Brown Brown
|
GreenLight greenLight greenBlackish green
Blackish green No change Blackish green Blackish green No change No change Blackish brown Blackish brown No change
|
Reddish orangeCherry redYellowRed
Purple-orange Reddish orange Red Violet- blood red Reddish orange Yellow Brick red Reddish orange Brick red
|
Dark yellowDark yellowYellowWine red
Violet Greenish yellow Dark orange Brownish yellow Dark orange Yellow Greenish yellow Greenish brown Greenish yellow
|
The above discussion about the colour reactions of chalcones shows that alcoholic FeCl3 gives colour reaction only with hydroxy chalcones while conc. H2SO4 – conc. HNO3 reagent provides colours due to the nitration of A-ring of chalcone molecule at 3′-position. NaBH4–HCl and Ac2OH2SO4 acid tests for chalcone show that the chromogen involved is Ar-CO–CH=CH–Ar and the reaction involves the attack by acetyliam ions at the carbonyl of the above chromogen giving —C+— (OAc)—CH = CH—.
Reference
- Kostanecki S.V. and Tambor J., Chem. Ber., 32: 1921(1899).
- Kostanecki S.V. and Rossbach G., Chem. Ber., 29: 1488 (1896).
- Hermans P.H., Theoritical Organic Chemistry, (Translation by S. Coffey), First English Edition, Elsevier Publishing Company, New York, 199 (1954).
- Weinland R., Einfuhrung in die chemie der komplex verbindungen, Second Edition, Stuttgart (1924).
- Peach K. and Fracey M.v., In Modern Methods of plant analysis, Springer–Verlag, 470 (1955).
- Reddelien G., Chem Ber., 45: 2904 (1912).
- Bagade M.B. and Ghiya B.J., Indian J.Chem., 30B: 71–74 (1991).
- Dhar B.N., Jashi S. and Dwevedi P., Indian J. Chem., 26B: 539 (1987).
- King H.G.C., White T. and Hughes R.B., J. Chem. Soc., 3234 (1961).
- Krishnamurthy H.G. and Seshadri T.R., Current science, 24: 681–685 (1965).
- Willson C.W., J. Am. Chem. Soc., 61: 2303-6 (1939).
- Anderson J.R., O’ Brien K.G. and Reuter F.H., Anal.Chem. Acta, 7: 226 (1952).
- Rangaswami S. and Seshadri T.R., Proc. Indian Acad. Sci., 16A: 129 (1942).
- Pfeiffer P., Fischer Ph., Kuntner J., Monti P. and Pros Z., Ann., 398: 137–96 (1913).
- PfeifferP., Organische Molekulverbindungen, Stuttgart: Ferd. Enke, M.135, 52.
- Marini-Bottolo G.B and Ballio A., Gazz. Chim, ital., 76: 410–18 (1946).

This work is licensed under a Creative Commons Attribution 4.0 International License.









