Synthesis and Characterization of Newly Synthesized Thiosemicarbazone Ligands with IR, NMR and Mass Spectral Techniques
N. Rama Jyothi* and N. A. Mohamed Farook
and N. A. Mohamed Farook
Department of Chemistry, Khadir Mohideen College, Adirampattinam, Tamilnadu, 614701-India.
Corresponding Author E-mail: ramadasaradhi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/35Specialissue106
Article Received on : 01-11-2018
Article Accepted on : 18-03-2019
Article Published : 21 Mar 2019
Among various organic chelating agents thiosemicarbazones occupy an important role due to their efficiency towards many metal ions through thionate sulphur atom and hydrazino nitrogen atom. Thiosemicarbazones can be obtained by condensing thiosemicarbazide with various carbonyl compounds. In recent years, the usage of thiosemicarbazone ligands has been reported by so many authors in the fields of analytical and biological. The present study reported about of two newly synthesized thiosemicarbazone ligands, namely 2,3,4-trihydroxybenzaldehyde-4-phenylthiosemicarbazone (THBPTSC) and 2,3,4-trihydroxybenzaldehyde-4-methylthiosemi carbazone (THBMTSC). These two new ligands are characterized with various spectral techniques, such as FT-IR, NMR and mass spectral techniques. The elemental composition was checked with elemental analysis report. Molar conductivity measurements of the ligands also reported.
KEYWORDS:FT-IR; NMR and Mass Spectra; Thiosemicarbazones; 2,3,4-Trihydroxybenzaldehyde-4-Phenylthiosemicarbazone; 2,3,4-Trihydroxybenzaldehyde-4-Methylthiosemicarbazone
Download this article as:| Copy the following to cite this article: Jyothi N. R, Farook N. A. M. Synthesis and Characterization of Newly Synthesized Thiosemicarbazone Ligands with IR, NMR and Mass Spectral Techniques. Orient J Chem 2019;35(Special Issue 1 Spectroscopy March 2019). |
| Copy the following to cite this URL: Jyothi N. R, Farook N. A. M. Synthesis and Characterization of Newly Synthesized Thiosemicarbazone Ligands with IR, NMR and Mass Spectral Techniques. Orient J Chem 2019;35(Special Issue 1 Spectroscopy March 2019). Available from: https://bit.ly/2W98FS4 |
Introduction
Organic chelating agents play a vital role in both analytical and biological fields. Among the various organic chelating agents, thiosemicarbazones are very important groups because of their wide applications. Thiosemicarbazones are having a great biological activities due to their ability to coordinate to the metal centers in enzymes. A number of studies reveals the biological and pharmacological activities of thiosemicarbazones and their metal complexes, such as antimicrobial, antimalarial, antiviral, anti-tumor and anti-HIV activities.
In recent years so many authors reported biological and analytical applications of thiosemicarbazones and/or their metal complexes. Salsi et al.1 reported anti-trypanosoma activity of thiosemicarbazones derived from fluorinated benzoylthioureas. Sens et al.2 reported the synthesis of new thiosemicarbazones which were acted as inhibitors of Mycobacterium tuberculosis protein tyrosine phosphate A. Deng et al.3 reported the anti-cancer property of copper complexes of 2-pyridine thiosemicarbazone ligands. A few studies4-7 reported about the analytical and biological properties of thiosemicarbazones. Antimicrobial activity of thiosemicarbazones and their metal complexes were studied by Ibrahim et al.8,9 and Temraz et al.10 Kalinowski et al.11 was reported the importance of thiosemicarbazones in cancer treatment. Matesanz and Souza12 reviewed about the antitumor activity of thiosemicarbazone ligands.
This paper describes synthesis and characterization of two new ligands namely, 2,3,4-trihydroxybenzaldehyde-4-phenylthiosemicarbazone (THBPTSC) and 2,3,4-trihydroxybenzalde -hyde-4-methylthiosemicarbazone (THBMTSC). These newly synthesized chelating agents were characterized with elemental analysis, molar conductivity measurement, FT-IR, NMR, and mass spectra.
Experimental
Chemicals
The chemicals used in this study was all are pure analytical grade. A Fisher-scientific stirrer was used for the stirring of the reactants which contains a hot plate.
Synthesis Scheme for 2,3,4-trihydroxybenzaldehyde-4-phenylthiosemicarbazone (THBPTSC)
Methanolic solutions of 1.54 g of 2,3,4-trihydroxybenzaldehyde (M.Wt. 303.35 g) in 100 mL and 1.67 grams of 4-phenylthiosemicarbazide (M.Wt. 105.16 g) in 100 mL were refluxed nearly 4h in a round bottom flask. The resultant product (yield, 79%) was separated by filtration and then it was dried. The final product was recrystallized once again from methanol. The synthesis scheme of THBPTSC is presented below (Scheme 1).
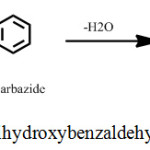 |
Scheme 1: Synthesis scheme of 2,3,4-trihydroxybenzaldehyde-4-phenylthiosemicarbazone. |
Synthesis of 2,3,4-trihydroxybenzaldehyde-4-methylthiosemicarbazone (THBMTSC)
Synthesis of THBMTSC is presented in Scheme 2. THBMTSC was synthesized by the refluxing of 1.54g of 2,3,4-trihydroxybenzldehyde (M.Wt. 303.35g) in methanol and 1.05 g of 4-methyl-3-thiosemicarbazide (M.Wt. 105.16 g) in methanol for nearly 4 h 30 min. in a round bottom flask. The resultant product (yield, 84%) was separated by filtration and then dried. The final product was recrystallized once again with methanol.
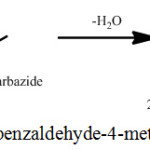 |
Scheme 2: Synthesis of 2,3,4-trihydroxybenzaldehyde-4-methylthiosemicarbazone. |
Characterization of Thiosemicarbazones with Elemental Analysis, FT-IR, NMR, and Mass Spectra
Elemental analysis (C,H,N and S) of THBPTSC and THBMTSC were performed on Thermo Scientific elemental analyzer (Thermo Eager 300 Flash EA1112, USA). Molar conductivity studies were performed with portable molar conductivity meter (MT-115, Manti Lab Solutions, India). FT-IR spectra were recorded on a Nicolet FT-IR 560 Magna spectrometer. High Resolution FAB mass spectra were recorded on a JMS 700 mass spectrometer (JEOL, Tokyo, Japan).
Results and Discussion
The synthesized thiosemicarbazone ligands, THBPTSC and THBMTSC are characterized with elemental analysis, molar conductivity measurements and with various spectral techniques, such FTIR, NMR and mass spectra.
Elemental Analysis
Elemental analysis reports of both the chelating agents, THBPTSC and THBMTSC are presented in Figures 1 and 2. The calculated elemental analysis data of THBPTSC (C14H13N3SO3) is C: 55.38%; H: 4.32%; N: 13.84%; S: 10.55%; O: 15.32%. This calculated data is well coincide with the practically obtained values as C: 54.58%; H: 4.31%; N: 12.89%; and S: 11.56% to above mentioned formula of THBPTSC. The theoretically calculated composition for TCMTSC (C9H11N3SO3), (C: 44.76%; H: 4.58%; N: 17.40%; S: 13.26% and O: 19.89%) is well in agreement with the practically found data (C: 45.22%; H: 4.58%; N: 16.33% and S: 15.29%)to the above proposed formula for THBMTSC.
Molar Conductivity Measurements
Molar conductivity values of THBPTSC and THBMTSC are 27.5 and 30.0 S cm2 mol-1, respectively. This indicates both ligands are having similar conductivity values.
 |
Figure 1: Elemental analysis report of THBPTSC. |
 |
Figure 2: Elemental analysis report of THBMTSC. |
FT-IR Spectral Analysis
FT-IR spectrum of THBPTSC is presented in Figure 3. The FT-IR data of THBPTSC is as follows: C=N peak appears at 1609 cm-1, C=S peak appears at 1255 cm-1and -NH peak appears at 3400 cm-1. This obtained data confirms the formation of the newly synthesized ligand, THBPTSC. FT-IR spectrum of THBMTSC is presented in Figure 4. FT-IR data of THBMTSC is as follows, C=N peak appears at 1630 cm-1 C=S peak appears at 1248 cm-1 and -NH peak appears at 3250 cm-1. This obtained data confirms the formation of the newly synthesized ligand, THBMTSC.
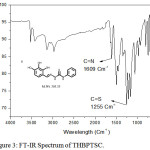 |
Figure 3: FT-IR Spectrum of THBPTSC. |
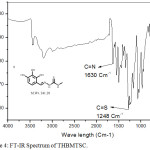 |
Figure 4: FT-IR Spectrum of THBMTSC. |
Proton NMR Spectral Characterization
Proton NMR spectrum data (DMSO/TMS) of THBPTSC is obtained as: 1H NMR (300 MHz, DMSO-d6): d=11.580 (s, 1H), 9.91 (s, 1H), 9.53 (s, 1H), 9.03 (broad, 1H), 8.45 (s, 2H), 8.34 (s, 1H), 7.57 (d, 1H), 7.33 (m, 2H), 7.16 (t, 1H), 6.35 (d, 1H). Based on the obtained proton NMR data it is clear that the formation of THBPTSC. The proton NMR spectrum of THBPTSC is presented in Figure 5. The proton NMR spectrum (DMSO/TMS) of THBMTSC is obtained as: d=11.22 (s, 1H; Ar-N-NH), 9.49 (broad, 1H; Ar-OH), 8.98 (broad, 1H; Ar-OH), 8.42 (broad, 1H; Ar-OH), 8.25 (m, 2H; CH3NH and Ar-CH), 7.14 (d, J=8.4 Hz, 1H; Ar), 6.34 (d, J= 8.4 Hz, 1H; Ar), 2.98 (d, 3H; NCH3). Based on the obtained proton NMR data it is clear that the formation of THBMTSC. The proton NMR spectrum of THBPTSC is presented in Figure 6.
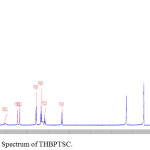 |
Figure 5: Proton NMR Spectrum of THBPTSC. |
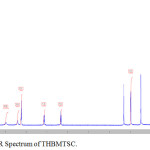 |
Figure 6: Proton NMR Spectrum of THBMTSC. |
Mass Spectral Characterization of Ligands
Based on molecular formula the molecular weight of THBPTSC is calculated as 303. The molecular weight obtained practically with mass spectrum as 304. Based on molecular formula the molecular weight of THBMTSC is calculated as 241. The molecular weight obtained practically with mass spectrum as 242. The mass spectra of THBPTSC and THBMTSC are presented in Figures 7, 8, respectively.
 |
Figure 7: Mass Spectrum of THBPTSC. |
 |
Figure 8: Mass Spectrum of THBMTSC. |
Conclusion
This study confirms the formation of two new thiosemicarbazone chelating compounds namely, 2,3,4-trihydroxybenzaldehyde-4-phenylthiosemicarbazone (THBPTSC) and 2,3,4-trihydroxy benzaldehyde-4-methylthiosemicarbazone (THBMTSC). The formation of these two new thiosemicarbazone chelating compounds was confirmed by the elemental analysis studies and various spectral analysis techniques, as FT-IR, 1H NMR and mass. These organic chelating agents can be used for the chelation with Cu(II) metal ions. The biological activities of both the chelating agents and their metal complexes will be evaluated in our future studies.
References
- Salsi, F.; Portapilla, G.B.; Schutjajew, K.; Carneiro, Z.A.; Hagenbach, A.; Albuquerque, S.; Maia, P.I.D.; Abram, U. J. Fluorine Chem., 2018, 215, 52-61.
- Sens, L.; De Souza, A.C.A.;Pacheco, L.A.; Menegatti, A.C.O.; Mori, M.; Mascarello, A.; Junes, R.J.; Terenzi, H. Bioorg.Med. Chem., 2018, In press.
- Deng, J.; Yu, P.; Zhang, Z.; Wang, J.; Cai, J.; Wu, N.; Sun, H.; Liang, H.; Yang, F. European J. Med. Chem., 2018, 158, 442-452.
- Haldys, K.; Goldeman, W.; Jewginski, M.; Wolirinska, E.; Anger, N.; Rossowska, J.; Latajka, R. Bioorg. Chem., 2018, 81, 577-586.
- Kallus, S.; Uhlik, L.; Schoonhoven, S.V.; Pelivan, K.; Berger, W.; Enyedy, E.A.; Hofmann, T.; Hffeter, P.; Kowol, C.R.; Keppler, B.K. J. Inorg. Biochem., 2019, 190, 85-97.
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Sahin, O.; Yalcin, B.; Sujayev, A.; Orman, E.B.; Ozkaya, A.R.; Gulcin, I. Polyhedron 2018, 155, 25-33.
- Zhang, H.H.; Chen, Y.; Zhang, A. Results in Physics 2018, 11, 554-563.
- Ibrahim, A.B.M.; Farh, M.K.; Mayer, P. Inorg. Chem. Commun., 2018, 94, 127-132.
- Ibrahim, A.B.M.; Farh, M.K.; El-Gyar, S.A.; EL-Gahami, M.A.; Fouad, D.M.; Silva, F.; Santos, I.C.; Paulo, A. Inorg. Chem. Commun., 2018, 96, 194-201.
- Temraz, M.G.; Elzahhar, P.A.; Bekhit, A.E.D.; Bekhit, A.A.; Labib, H.F.; Belal, A.S.F. Eur.J . Med. Chem., 2018, 151, 585-600.
- Kallnowski, D.S.; Quach, P.; Richardson, D.R. Future Med. Chem., 2009, 1(6), 1143-1151.
- Matesanz, A.; Souza, P. Mini Rev. Med. Chem., 2009, 9(12), 1389-1396.

This work is licensed under a Creative Commons Attribution 4.0 International License.









