Study of Inhibition and Adsorption Properties of Mild Steel Corrosion by Expired Pharmaceutical Gentamicin Drug in Hydrochloric Acid Media
A. Srinivasulu1 and P. K. Kasthuri2
1Research and Development Centre, Bharathiar University, Coimbatore, Tamil Nadu, India.
2Department of Chemistry, L.R.G.Government Arts College for Women, Tirupur, Tamil Nadu, India.
Corresponding Author E-mail: achus.aslu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330559
This experiment promotes economically, eco-friendly benefitted inhibitor and also avoids pollution and recycles the drugs. In this experiment expired Gentamicin drug was tested its inhibitive effect in 1 M HCl acid medium on mild steel, with the electrochemical and weight loss techniques. The Various parameters were calculated such as inhibition efficiency, corrosion rate and surface coverage. The inhibition efficiency shows increases when inhibitor concentration, immersion period and temperature increase in weight loss method. Obtained results in electrochemical Impedance and weight loss studies are very much excellent agreement with each other. Thermodynamic parameter free energy value was negative, it shows spontaneous adsorption of inhibitor on the surface of the mild steel. The adsorption nature on the surface of mild steel was under conformity by Langmuir adsorption isotherm. The surface morphology, with and without inhibitor was studied with using scanning electron microscopy.
KEYWORDS:Mild steel, acidic medium; economically benefitted corrosion inhibitor; expired-drug; electrochemical Impedance and weight loss methods; the mild steel surface morphology
Download this article as:| Copy the following to cite this article: Srinivasulu A, Kasthuri P. K. Study of Inhibition and Adsorption Properties of Mild Steel Corrosion by Expired Pharmaceutical Gentamicin Drug in Hydrochloric Acid Media. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Srinivasulu A, Kasthuri P. K. Study of Inhibition and Adsorption Properties of Mild Steel Corrosion by Expired Pharmaceutical Gentamicin Drug in Hydrochloric Acid Media. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=36720 |
Introduction
Simple accessibility, minimal effort of mellow steel has numerous industrial applications. It is broadly utilized as a part of different ventures like sugar, cowhide, sustenance, petrochemical, paper and material businesses. In most mechanical procedures, the acidic arrangements are regularly utilized for the pickling, acid cleaning, de-scaling of acids etc. The decreasing corrosion rate of metals spares the current assets and thusly creates conservative advantages amid the mechanical applications. The lifetime of types of gear can be expanded and viable corrosion restraint will diminish the disintegration of harmful metals from the parts into nature [1-3]. Hydrochloric acid is most the most corrosive with most of the common metals and alloys, difficult of this acid to handle from corrosion and materials of constructions, due to its industrial applications the corrosion inhibitors are became an answer for corrosion attack witch lead to metal damage and also replacement of the metal. Many reviews on consumption inhibitors tell that a large portion of the inhibitors are organic with N, O and S atoms or with polar groups of N-hetero cyclic compounds. They have fundamental properties with high electron thickness, making them the response focuses [4-10].These block the dynamic corrosion locations by adsorption on the metallic surface and the majority of them are exceedingly dangerous to the people and also the earth. Thus replacement by these eco-friendly inhibitors is necessary. The use of pharmaceutical compounds which are contains hetero atoms in their structure, high solubility and high molecular weight offers interesting possibilities for corrosion inhibition [11-20]. Few drugs like azosulpha and antimalarial have been accounted for that they are great corrosion inhibitors on mild steel [21-23]. In this studies Gentamicin drug (Eye/Ear Drops) that has been expired is chosen for the corrosion inhibition on the mild steel in 1M hydrochloric acid medium on the mild steel by using weight loss, electrochemical spectroscopy strategies. Gentamicin is a non-toxic pharmaceutical medication used to prevent or treat a wide variety of bacterial infections. It belongs to a class of drugs known as amino glycoside antibiotics, it contain N- atom having high electron density to block the active sites of corrosion. The inhibitor is available in the brand name of ‘Gentamicin’ fabricated in India by CIPLA LTD it works as an anticorrosive agent in hydrochloric acid medium on mild steel.
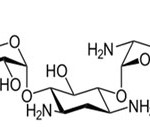 |
Scheme 1 |
Experimental
Materials
Mild steel strips are cut into segments of dimensions 5 cm x1 cm x 0.2cm, with a hole (2mm) which has a uniform diameter length at one of its end for hooking. The composition of strip is as follows: 0.256 % Mn, 0.034% C, 0.004 % P, 0.023 % Si and the remainder Fe. For studies in electrochemistry, these specimens of the same composition were made by fixing the mild steel of size 1 cm2 to a mild steel rod with a diameter 1mm utilizing araldite. Each piece was polished with emery paper, degreased with acetone and was washed with distilled water. After dried they are stored in the desiccators. Weight of these specimens (strips) was given utilizing electronic balance. Analar grade HCL acid and double time distilled water were utilized to make all the solutions. Expired Gentamicin (Eye/Ear Drops) drug obtained from medical shop and used for this study without any further purification.
Results and Discussion
Weight loss Method
Initial weights of the pretreated example’s are noted triplicate was inundated in the 100 ml (1M HCL) solution in nearness and nonappearance of the inhibitors at difference concentrations (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0%) for the period of 0.5, 2, 4, 6, 8 and 24 hours with the help of glass hooks after the experiment period the final weights were also noted and also studied impact of temperature on mild steel corrosion at five distinct temperatures ranging from 303K to 343K. From this the inhibition efficiency (IE %), rate of corrosion (mils Penetration per year), surface coverage (θ) were estimated by utilizing the following formulae.
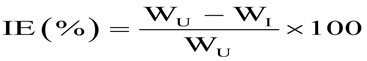
Where, WI and WU are weight losses in acids with and without inhibitors respectively.
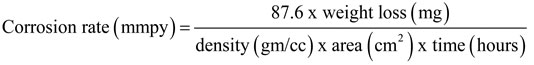
Corrosion parameters obtained from this method are given in Tables 1 & 2. It shows that, the inhibition efficiency incremented with the increase in temperature, concentration and immersion period of inhibitor as it is shown in the Figures 1 & 2. The maximum inhibition efficiency 92.36% was noticed in 6 hours immersion period at room temperature (303 K) with concentration of 0.9% .This outcome shows that the inhibitor could act as effective corrosion inhibitor for mild steel in 1M HCl medium.
Table 1: Influence by the concentration of expired Gentamicin inhibitor in corrosion of 1M HCL at the room temperature (303 K) with various time periods.
| Time | ½ hrs | 2 hrs | 4 hrs | 6 hrs | 8 hrs | 24 hrs | ||||||
| InhibitorConc.(%V/V) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) |
| Blank | 514.52 | 590.34 | 633.18 | 647.62 | 699.58 | 714.34 | ||||||
| 0.1 | 472.69 | 40.22 | 500.21 | 44.36 | 506.64 | 49.28 | 514.68 | 55.23 | 546.24 | 52.36 | 564.38 | 43.26 |
| 0.2 | 408.64 | 47.65 | 395.64 | 53.26 | 391.12 | 57.23 | 386.98 | 60.21 | 402.82 | 58.65 | 445.67 | 49.68 |
| 0.3 | 321.76 | 55.36 | 314.06 | 60.25 | 306.16 | 64.65 | 293.19 | 67.64 | 308.19 | 65.23 | 348.68 | 58.31 |
| 0.4 | 249.08 | 63.21 | 238.18 | 66.21 | 229.06 | 69.36 | 221.98 | 72.63 | 240.68 | 70.26 | 279.98 | 62.35 |
| 0.5 | 187.23 | 65.23 | 180.64 | 68.65 | 174.33 | 72.25 | 166.82 | 75.34 | 184.96 | 73.65 | 223.14 | 67.32 |
| 0.6 | 146.72 | 67.54 | 138.21 | 71.26 | 130.28 | 74.65 | 123.04 | 78.26 | 142.08 | 76.31 | 180.86 | 69.11 |
| 0.7 | 138.66 | 69.28 | 127.82 | 74.63 | 124.67 | 78.56 | 102.98 | 83.69 | 126.82 | 79.98 | 139.68 | 73.01 |
| 0.8 | 124.28 | 73.02 | 121.36 | 77.65 | 115.29 | 83.99 | 98.89 | 88.69 | 111.21 | 85.02 | 125.68 | 76.99 |
| 0.9 | 119.66 | 76.65 | 112.99 | 80.36 | 106.28 | 86.23 | 95.29 | 92.36 | 107.08 | 88.55 | 116.69 | 80.64 |
| 1.0 | 126.78 | 74.36 | 118.87 | 78.21 | 116.72 | 83.26 | 110.88 | 87.66 | 116.66 | 85.06 | 146.95 | 74.66 |
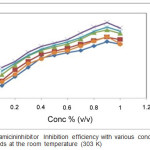 |
Figure 1: Gentamicin inhibitor Inhibition efficiency with various concentrations and time periods at the room temperature (303 K). |
Table 2: Temperature effect on mild steel corrosion in the presence of various concentrations of Expired Gentamicin inhibitor in 1M HCL
| Temperature | 303K | 313K | 323K | 333K | 343K | |||||
| InhibitorConc.(%V/V) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) | CR(mpy) | IE(%) |
| Blank | 514.52 | 1539.18 | 3519.25 | 8109.48 | 16527.78 | |||||
| 0.1 | 472.69 | 40.22 | 1006.36 | 44.28 | 2432.69 | 50.36 | 5257.26 | 55.65 | 11063.23 | 48.65 |
| 0.2 | 408.64 | 47.65 | 821.63 | 52.64 | 1802.69 | 59.82 | 3806.23 | 65.63 | 7706.21 | 56.32 |
| 0.3 | 321.76 | 55.36 | 563.21 | 60.98 | 1698.31 | 65.32 | 2722.61 | 69.36 | 5423.65 | 63.21 |
| 0.4 | 249.08 | 63.21 | 390.23 | 68.36 | 1230.65 | 72.16 | 1960.23 | 74.32 | 3994.25 | 68.65 |
| 0.5 | 187.23 | 65.23 | 269.32 | 72.98 | 893.22 | 76.21 | 1426.33 | 78.61 | 2904.66 | 73.63 |
| 0.6 | 146.72 | 67.54 | 188.78 | 75.36 | 647.23 | 79.45 | 999.69 | 82.65 | 1725.36 | 77.65 |
| 0.7 | 138.66 | 69.28 | 169.08 | 78.22 | 499.29 | 82.01 | 785.25 | 85.65 | 1529.99 | 80.65 |
| 0.8 | 124.28 | 73.02 | 162.55 | 80.11 | 466.21 | 85.75 | 726.32 | 87.65 | 1389.45 | 82.31 |
| 0.9 | 119.66 | 76.65 | 158.36 | 82.65 | 438.62 | 87.06 | 665.32 | 89.31 | 1198.32 | 84.65 |
| 1.0 | 126.78 | 74.36 | 166.32 | 78.23 | 528.95 | 83.22 | 869.66 | 86.32 | 1456.01 | 80.65 |
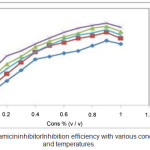 |
Figure 2: Gentamicin inhibitor Inhibition efficiency with various concentrations and temperatures. |
Potentiodynamic Polarization Method
Electrochemical analyzer was used for Potentiodynamic polarization measurements. These estimations were concluding to assess the corrosion potential, corrosion current and Tafel slopes. A conventional three electrode cell assembly which contains working electrode as the mild steel specimen with area 1 cm2 which is exposed and rest covered with araldite, as counter electrode rectangular Pt foil was used and the SCE as reference electrodes. To get the steady state open circuit potential for each test, ten to thirty minute’s time interval was given. From the cathode with potential of -450mV (vs. SCE) to an anode with potential of -250mV (vs. SCE) at a sweep rate of 10mV per second polarization measurements were given. Corrosion potential, Tafel slopes and corrosion rates were calculated with polarization curves. The inhibitor efficiency was given by utilizing the following formulas.
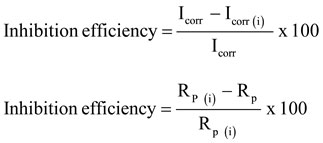
Where, Icorr and Icorr (i) are the corrosion current in absence of the inhibitor and presence of the inhibitor, similarly Rp and Rp (i) are the polarization resistance in the absence and presence of the inhibitor.
Polarization studies observed that when concentration of inhibitor increased from 0.4% to 0.9% the Icorr values decreased 0.996 mA/cm2 to 0.249 mA/cm2 with inhibition value increase from 64.16% to 75.06% and also (Rp) values increased from 18.92Ωcm2 to 73.15Ωcm2 with increase of inhibition efficiency from 65.51% to 74.13%. Tafel polarization behaviors of the mild steel 1M HCL shows in the Figure 3. From the graph we can observe that the inhibitor behaves like mixed type [24-28]. In the Table 3.all the calculated data of Polarization studies was given.
Table 3: Electrochemical polarization (Tafel) parameters for mild steel corrosion in 1M HCL without and with various concentrations of inhibitor at room (303 K) temperature.
| conc.(%) | -Ecorr (mV) | I corr(mA/cm2) | Icorr | ba (mV/dec) | bc(mV/dec) | Rp(Ωcm2) | Rp |
| IE (%) | IE (%) | ||||||
| blank | 346.8 | 0.996 | – | 70.0 | 114 | 18.92 | – |
| 0.4 | 306.0 | 0.355 | 64.16 | 77.0 | 107 | 54.86 | 65.51 |
| 0.7 | 304.5 | 0.329 | 66.90 | 70.0 | 110 | 56.27 | 66.38 |
| 0.9 | 305.1 | 0.249 | 75.06 | 68.0 | 109 | 73.15 | 74.13 |
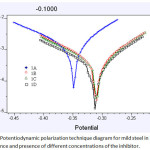 |
Figure 3: Potentiodynamic polarization technique diagram for mild steel in 1M HCl in the absence and presence of different concentrations of the inhibitor. |
1A=BLANK, 1B=0.4%, 1C=0.7%, 1D=0.9%
Electrochemical Impedance Methods
The same cell as utilized which potentiodynamic polarization in the calculations of electrochemical impendence, An AC potential 50 mV was super forced on the consistent open circuit potential. The genuine part (Z’) and the fanciful part (Z”) were measured at different frequencies in the scope of 10 kHz to 10MHz. The genuine and imaginary parts of the impendence were plotted in Nyquist plots. From the plots of Z’ versus Z,” the charge transfer resistance (Rct) quantities were acquired. The estimation of (Rt + Rs) compares to the point where the plot cuts z’ and at a higher frequency the distinction amongst Rt and Rs gives the charge exchange resistance Rct values. The double layer capacitance Cdl quantities were acquired from the following condition
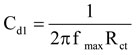
Where, Cdl means double layer capacitance
Rct means charge transfer resistance
fmax means frequency at Z” maximum value .
The inhibition efficiencies were calculated from Rp and Rct quantities as follows
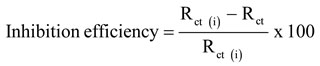
Where, Rct and Rct (i) are charge transfer resistance in the absence of inhibitor and presence of the inhibitor Nyquist plots shows in the Figure 4 for the measurements of the genuine part (Z’) and imaginary one (Z”). We can conclude the corrosion is mostly controlled by the process of charge transfer from semi-circle curves of the impedance. In Table 4, parameters for impedance of mild steel in 1M HCL in the presence of inhibitor and absence of the inhibitor are shown. The values of Rct had increased from 3.43 Ωcm2 to 15.91 Ωcm2 and simultaneously the values of Cdl had decreased 438 μF/Cm2 to 88 μF/Cm2 with the increase in inhibitor concentrations.
Table 4: Electrochemical impedance parameters for the mild steel corrosion in 1M HCL without and with various concentrations of inhibitor at room (303 K) temperature.
| Conc.(%) | Rct(Ωcm2) | CdlµF/cm2 | IE(%) |
| blank | 3.43 | 438 | – |
| 0.4 | 9.21 | 132 | 62.76 |
| 0.7 | 12.95 | 164 | 73.51 |
| 0.9 | 15.91 | 106 | 78.44 |
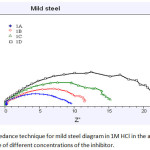 |
Figure 4: Impedance technique for mild steel diagram in 1M HCl in the absence and presence of different concentrations of the inhibitor. |
1A=BLANK, 1B=0.4%, 1C=0.7%, 1D=0.9%
Thermodynamic Consideration
From the following Arrhenius equation the activation energy with different concentrations at various temperatures was calculated.
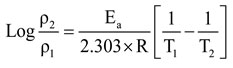
Where, ρ1 is the corrosion rate at T1 and ρ2 is the corrosion rate at T2 temperature. And ‘R’ is the real gas constant.
The change in the free energy at various temperatures compared to room temperature in various concentrations of the inhibitor was calculated by the following equation
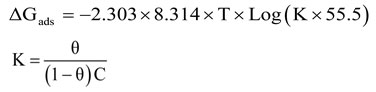
Where, Surface coverage (θ), Concentration (C), Temperature (T) & Equilibrium constant (K).
A change in enthalpy of adsorption (∆H) and a change in entropy of adsorption (∆S) can be calculated by utilizing the following equations.
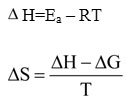
Values of activation energy (Ea), free energy (∆Gads), the entropy (∆S) and the enthalpy (∆H) for mild steel in 1M HCl acid in the presence and the absence of the inhibitor are calculated and are shown in Table 5. Value of activation energy (Ea) of blank is 73.83 kJ mol-1 and 51.66 kJ mol-1 for 0.9% inhibitor concentration, it’s now clear that the addition of the inhibitor leads to decrease in the obvious activation energy (Ea) with some value that is lesser than the value of the uninhibited solution which is trailed by the dull decline with the rise in the concentration of the inhibitor which points out the action upon mild steel in the 1M HCl acid takes place through via the chemical adsorption. The (-ve) value of the free energy of adsorption (∆Gads) points out adsorption which would be spontaneous. (+ve ) values of enthalpy recommend that high temperatures will favor the complexation activity and same in with a fantastic agreement with the incrementing firmness with temperature. The negative estimations of ΔSact indicated a greater order which is generated during the activation process. This can be accomplished by the creation of initiated complex and it shows the affiliation or fixation with the resulting decrease in the degrees of the freedom of framework in this procedure. This also supports supposition of the chemical adsorption. Figure 5 illustrates the Arrhenius plot for mild steel dissolution in 1M HCl acid in the presence and the absence of the inhibitor at various temperatures.
Table 5: Thermodynamic data for mild steel corrosion in 1M HCL in the presence and absence of Expired Gentamicin inhibitor in 1M HCL for a period of ½ hour.
| InhibitorConc.(%V/V) | Ea (KJ/mol) | – ΔG (KJ/mol) | -ΔS(KJ/mol) | ΔH(KJ/mol) | |||||||||
| Temp | θ | 303K | θ | 313K | θ | 323K | θ | 333K | θ | 343K | 303K | 303K | |
| blank | 73.83 | 71.31 | |||||||||||
| 0.1 | 67.97 | 0.402 | 14.92 | 0.442 | 15.34 | 0.503 | 15.95 | 0.556 | 16.49 | 0.486 | 15.78 | 0.265 | 65.45 |
| 0.2 | 63.36 | 0.476 | 13.93 | 0.526 | 14.44 | 0.598 | 15.17 | 0.656 | 15.80 | 0.563 | 14.81 | 0.246 | 60.84 |
| 0.3 | 61.92 | 0.553 | 13.69 | 0.609 | 14.27 | 0.653 | 14.74 | 0.693 | 15.21 | 0.632 | 14.51 | 0.241 | 59.4 |
| 0.4 | 61.33 | 0.632 | 13.79 | 0.683 | 14.36 | 0.721 | 14.82 | 0.743 | 15.10 | 0.686 | 14.40 | 0.239 | 58.81 |
| 0.5 | 61.16 | 0.652 | 13.45 | 0.729 | 14.36 | 0.762 | 14.79 | 0.786 | 15.14 | 0.736 | 14.45 | 0.237 | 58.64 |
| 0.6 | 56.45 | 0.675 | 13.25 | 0.753 | 14.22 | 0.794 | 14.81 | 0.826 | 15.33 | 0.776 | 14.54 | 0.221 | 53.93 |
| 0.7 | 54.09 | 0.692 | 13.06 | 0.782 | 14.23 | 0.820 | 14.84 | 0.856 | 15.51 | 0.806 | 14.61 | 0.213 | 51.57 |
| 0.8 | 54.03 | 0.730 | 13.19 | 0.801 | 14.19 | 0.857 | 15.20 | 0.876 | 15.61 | 0.823 | 14.55 | 0.213 | 51.51 |
| 0.9 | 51.66 | 0.766 | 13.38 | 0.826 | 14.31 | 0.870 | 15.18 | 0.893 | 15.73 | 0.846 | 14.68 | 0.206 | 49.14 |
| 1.0 | 55.91 | 0.743 | 12.80 | 0.782 | 13.34 | 0.832 | 14.15 | 0.863 | 14.76 | 0.806 | 13.71 | 0.218 | 53.39 |
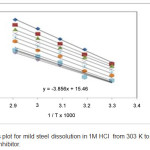 |
Figure 5: Arrhenius plot for mild steel dissolution in 1M HCL from 303K to 343 K temperature with and without inhibitor. |
Adsorption Consideration
Corrosion Inhibition is recognized with inhibitor adsorption molecules on the metal surface. Surface coverage (θ) is equal to IE/100. In order to find out the adsorption isotherm for the present review, a plot C/θ and C ought to be linear. This demonstrates that the adsorption takes place after the Langmuir adsorption isotherm. These isotherms give the best fit with the correlation co efficient almost near to (0.9989) unity. In Figure 6 shows a straight line showing Langmuir adsorption isotherm. Organic molecules contain polar atoms which are adsorbed on the surface of the metal and interact with mutual repulsion or attraction. Because this plot is linear, gradients never unite, Langmuir adsorption isotherm is shown as:
C/θ=1/K + C
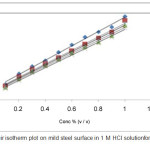 |
Figure 6: Langmuir isotherm plot on mild steel surface in 1 M HCL solution for the adsorption of inhibitor. |
Morphology Examination
The pictures of mild steel in the acidic medium containing without and with the inhibitor are shown in Figures 7 & 8. It is shows that the assault of the mild steel within the existence of inhibitor in 1 M HCl is very less compared to the nonappearance of inhibitor, because of the presence of adsorbed layer of inhibitor on the surface which blocks corrosion rate of metal evidently. This is ascribed to the contribution of compounds of the inhibitor in interaction with the active sites on surface of the metal, thus gives enhanced surface coverage of metal so that there is a decrease in contact amongst aggressive medium and the metal.
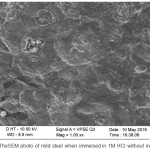 |
Figure 7: The SEM photo of mild steel when immersed in 1M HCL without inhibitor. |
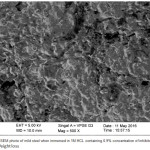 |
Figure 8: The SEM photo of mild steel when immersed in 1M HCL containing 0.9% concentration of inhibitor. |
Table 6: The inhibition efficiency competition of inhibitor in 1M HCl for mild steel from weight loss, polarization and impedance techniques for the period of ½ hour studies.
| Conc.(%) | Inhibition efficiency (%) | |||
| Weight loss | Polarization | Impedance | ||
| Icorr | Rp | |||
| 0.4% | 63.21 | 64.16 | 65.51 | 62.76 |
| 0.7% | 69.28 | 66.90 | 66.38 | 73.51 |
| 0.9% | 76.65 | 75.06 | 74.13 | 78.44 |
Conclusion
The expired Gentamicin (Eye/Ear drops) pharmaceutical drug acts as excellent, efficient and economically benefitted inhibitor for corrosion in the 1M HCl acid medium on mild steel. The efficiency of the inhibitor is increase with period of immersion, temperatures and concentration of inhibitor. The Inhibitor with concentration (0.9%) showed maximum efficiency (92.36%) in 6h immersion period at room temperature (303K) and found sufficient for pickling. The chemical adsorption mechanism of the inhibition process was conformation with the data obtained; Langmuir adsorptions were best fitted into the obtained results. Thermodynamic parameters like activation energy (Ea) values also conformed to a chemical adsorption mechanism. Free energy (Gads) of absorption values are negative which shows spontaneous adsorption of the inhibitor on the surface. The positive Enthalpy values are proposing high temperature favors inhibition efficiency. The corrosion is mainly controlled by phenomena of charge transfer by seeing semi circle curves of impedance. High performance of the inhibitive effect on the surface of mild steel has confirmed by SEM morphology of protective film. Obtained results in electrochemical and weight loss studies are very much excellent agreement with each other. This experiment promotes eco-friendly and economically benefitted inhibitor and also avoids pollution and recycles the drugs. Inhibition efficiency of inhibitor for mild steel in 1M HCl for three difference methods is given in the Table 6.
Reference
- Aejitha .S, Kasthuri .P. K and Geethamani , Corrosion Studies with Antigonon Leptopus In 1 M HCl, Int. J. Chem. Sci.: 2015,13(1), 38-52
- Amine .M.A and Ibrahim .M.M, corrosion and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative, Corros. Sci. 2011, 53, 873-885.
CrossRef - Ishtiaque .A, Rajendra .P and Quraishi .A.M, Inhibition of mild steel corrosion in acid solution by Pheniramine drug: Experimental and theoretical study, Corros. Sci. 2010, 52, 3033–3041
CrossRef - Bentiss .F, Bouanis .M M., Marwari .B, Traisnel .M, and Lagrenée .M.M, Effect of iodide ions on corrosion inhibition of mild steel by 3,5-bis(4-methylthiophenyl)-4H–1,2,4-triazole in sulfuric acid solution, J. Appl. Electrochem, 2002, 32, 671–678.
CrossRef - Fouda .S, Elewady .G.Y, Shalabi .K and Habouba .S, Tobacco Plant Extracts as Save corrosion Inhibitor for Carbon Steel in Hydrochloric Acid Solutions, IJAR, 2014, 2(3), 817-832
- Onuegbu .T.U, Umoh .E.T and Onuigbo .U.A, Eupatorium Odoratus As Eco-Friendly Green Corrosion Inhibitor of Mild Steel In Sulphuric Acid, International Journal Of Scientific & Technology Research, 2013,2(2), 4-8
- Quraishi .M.A, Dileep Kumar Yadav and Ishtiaque Ahamad, Green Approach to Corrosion Inhibition by Black Pepper Extract in Hydrochloric Acid Solution, The Open Corrosion Journal , 2009, 2, 56-60
CrossRef - Leelavathi .S and Rajalakshmi .R, Dodonaea viscosa (L.) Leaves extract as acid Corrosion inhibitor for mild Steel – A Green approach, J. Mater. Environ. Sci. 2013, 4(5), 625-638
- Priyaa .S.V and Saratha .R, Inhibition of mild steel corrosion in 0.5 H2SO4 with leaves extract, Elixir Corrosion, 2011 , 37 , 3617-3622
- Abdallah .M and El-Naggar .M.M, Cu+2 cation + 3, 5-dimethyl pyrazole mixture as a corrosion inhibitor for carbon steel in sulfuric acid solution, Mater. Chem. Phys., 2001, 71, 291-298.
CrossRef - Aejitha .S , Kasthuri .P.K, Inhibition Action of Mild Steel Corrosion in HCl Acid Medium by Extract of Digera Muricata, International Journal of Science and Research, 2014,3(9), 607- 611.
- Selvamani .P, Latha .S and Dhivya .P.S, Anti-Inflammatory Activity of Extracts of Commiphora Species and Its Polyherbal Formulation, International Journal of Phytopharmacology. 2013, 4(5), 288- 292
- SudarshanaDeepa .V, Suresh Kumar .P, Latha .S, Selvamani .P and Srinivasan .S, Antioxidant Studies on the Ethanolic Extract of CommiphoraSpp, African Journal of biotechnology, 2009, 8 (8), 1630-1636
- GeethaKodali and Ganapaty Seru, Antihyperlipidemic Activity of CommiphoraCaudataLeaves In Atherogenic Diet Induced Rats, International Journal of Biological & Pharmaceutical Research. 2013, 4(4), 250-255.
- Adeyemi .O.O and Olubomehin .O.O , Investigation of Anthocleistadjalonensis Stem Bark Extract as Corrosion Inhibitor for Aluminum, The Pacific Journal of Science and Technology, 2010,11(2), 455-462.
- Ehteram A. Noor, Temperature Effects on the Corrosion Inhibition of Mild Steel in Acidic Solutions by Aqueous Extract of Fenugreek Leaves, Int. J. Electrochem. Sci., 2007, 2, 996 – 1017
- Anbarasi .K and Vasudha .V.G , Corrosion Inhibition Potential of Cucurbita Maxima Plant Extract on Mild Steel in Acid Media, Chemical Science Review and Letters, 2014, 3(9), 45-51
- Ladha .D.G, Naik .U.J and Shah .N.K, Investigation of Cumin (CuminumCyminum) extract as an ecofriendly green corrosion inhibitor for pure Aluminium in Acid medium, J. Mater. Environ. Sci. 2013, 4 (5), 701-708
- Gunavathy .N and Murugavel .S.C, Study On The Effect Of Musa AcuminataFlower Extract On The Corrosion Inhibition Of Mild Steel In 1 N H2SO4, Int. J. Chem. Sc.: 2013, 11(1), 475-486
- Rajalakshmi .R, Subhashini .S, Leelavathi .S and Geethajali .R, Study Of The Inhibitive Action Of Bakery Waste For Corrosion Of Mild Steel In Acid Medium ,J. Nep. Chem Soc., 2010,25, 29-36 .
- Vasudha .V.G and Shanmuga Priya .K, PolyalthiaLongifoliaas a Corrosion Inhibitor for Mild Steel in HCl Solution, Research Journal of Chemical Sciences, 2013, 3(1), 21-26
- Priya .S.V and Saratha .R, Inhibition of mild steel corrosion in 0.5 H2SO4 with leaves extract, Elixir Corrosion, 2011, 37, 3617-3622
- Aouine .Y , Sfaira .M, EbnTouhami .M, Alami .A, Hammouti .B , Elbakri .M, El Hallaoui .A and Touir .R, Temperature and Time Investigations on the Adsorption Behavior of Isoindoline, Tetrazole and Isoindoline-Tetrazole on Corrosion of Mild Steel in Acidic Medium,Int. J. Electrochem. Sci., 2012, 7 , 5400 – 5419
- Sheeja .V.N and Subhashini .S, 2014, PavettaIndicaBark as Corrosion Inhibitor in Mild Steel Corrosion in HCl and H2SO4 Medium: Adsorption and Thermodynamic Study, Chemical Science Transactions, 2014, 3(1), 129-140
- SrinivasaReddy .C, Ammani.K, RoseMary.T, Nikhil Rajesh.D, Aravind.G, and ChandraBala Sekaran, Phytochemical and GC-MS analysis of Commiphoracaudata(Wt. &Arn.) Eng. Bark, Indian Journal of Advances in Plant Research, 2014, 1(5), 24-29.
- Martinez .S and Stern .I, Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system, Appl. Surf. Sci., 2002, 199 (1), 83– 89.
CrossRef - KhuloodA.saleh, Khalil S. Khalil, Corrosion Inhibition Of Zinc In Hydrochloric Acid Solution Using Ampicillin, Iraqi Journal of Science, 2014, 55, 295-303
- Ambrish Singh and Eno E. Ebenso, Use of Glutamine as a New and Effective Corrosion Inhibitor for Mild Steel in 1 M HCl Solution,Int. J. Electrochem. Sci., 2013, 8, 12874 – 12883
- Khadom .A.A , Yaro .A.S, AlTaie .S and Kadum .A.H, Electrochemical, Activations and Adsorption Studies for the Corrosion Inhibition of Low Carbon Steel in Acidic Media, PortugaliaeElectrochimicaActa , 2009, 27(6), 699-712.
- Sharmila .A, AngelinPrema .A and ArockiaSahayaraj .P, Influence Of MurrayaKoenigii (Curry Leaves) Extract On The Corrosion Inhibition Of Carbon Steel In HCl Solution, Rasayan .J.Chem., 2010, 3(1), 74- 81.

This work is licensed under a Creative Commons Attribution 4.0 International License.









