Uranium Sources, Uptake, Translocation in Soil-Plant System and its Toxicity in the Plants and Humans: A Critical Review
Sandeep Singh Duhan , Pradeep Khyalia
, Pradeep Khyalia , Pooja Solanki
, Pooja Solanki and Jitender Singh Laura*
and Jitender Singh Laura*
Department of Environmental Science, Maharshi Dayanand University Rohtak, Haryana, India
Corresponding Author E-mail: Jslmduu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/390210
Article Received on : 25 Feb 2023
Article Accepted on : 30 Mar 2023
Article Published : 06 Apr 2023
Reviewed by: Dr. Raja Solomon Viswas /a>
Second Review by: Dr. Pooja Deskmukh
Final Approval by: Dr. Bal krishan Sharma
Uranium(U) is one of the highly toxic heavy metals and radionuclides that has become a major threat to soil health. There are two types of sources of Uranium in the soil system, natural and anthropogenic. Natural sources of uranium include rock systems and volcanic eruptions while anthropogenic sources include mining activities, disposal of radioactive waste, application of phosphate fertilizers, etc. Uranium accumulation impacts germination, early seedling growth, photosynthesis, metabolic and physiological processes of the plants. Through its accumulation in the aerial parts of the plants, Uranium finds its way to the human body, where it has deleterious health impacts. Different studies have identified the various sources of Uranium, explored, and explained the geochemistry of Uranium in soil, assessed the Uranium uptake and toxicity to the plants, and further studied the impact on human health. Most studies focused on two stages, either soil-plant or plant-human system. However, few studies have critically reviewed and summarized the U in the soil-plant-human system. Thus, the review has been designed to focus on the sources, geochemical behaviour, uptake, and translocation, plant toxicity, food chain entry, and finally, impact on human health. The relationship between the bioavailability of Uranium in the soil-plant system with soil properties like pH, Organic matter, and microorganisms have also been included. The study is further intensified by analyzing the accumulation of Uranium in various parts of the plants.
KEYWORDS:Bioavailability; Health Impact; Plant; Soil; Translocation; Uranium
Download this article as:| Copy the following to cite this article: Duhan S. S, Khyalia P, Solanki P, Laura J. S. Uranium Sources, Uptake, Translocation in the Soil-Plant System and its Toxicity in Plants and Humans: A Critical Review. Orient J Chem 2023;39(2). |
| Copy the following to cite this URL: Duhan S. S, Khyalia P, Solanki P, Laura J. S. Uranium Sources, Uptake, Translocation in the Soil-Plant System and its Toxicity in Plants and Humans: A Critical Review. Orient J Chem 2023;39(2). Available from: https://bit.ly/3KhNurF |
Introduction
Uranium, a naturally radioactive element having an atomic number of 92 and an atomic weight of 238.03, was initially found as a part of pitchblende discovered by German chemist Martin Heinrich Klaproth in 1789 1. In its crystalline state, its valence varies from +3 to +6. Only the hexavalent uranyl compounds (UO22+) are thermodynamically and kinetically stable in an aqueous solution for biological activities. Uranium forms various oxides, such as UO2, U3O8, and UO3. Uranates are made by fusing uranium with carbonates available on earth. Uranium, a very lethal environmental contaminant, has gained considerable attention in the field of research due to its chemical and radiotoxicity. It has been discovered as a highly detrimental environmental contaminant for all living beings, including humans, and its chemical reactions and radiotoxicity make it a reason for toxicity to plants, animals, and humans 2,3.
Uranium is carcinogenic and a radioactive element 4,5 once its concentration increases above 0.05 mg/kg body mass 6. When Organisms ingest U, it has a long-term chemical toxicity effect 7. The entry of U into an organism via the food chain is hazardous 8. The most common way for U to enter the body is via drinking water. The suggested permissible limit of uranium for drinking water is 30µg/L, exceeding which could have long-term health consequences for humans 9. Both anthropogenic and geogenic activities influence the sources of elements in groundwater 10-13. Ingestion of groundwater containing high levels of U for a long time may affect bone and kidneys 14. The bioavailability of Uranium is inversely proportional to its chemical state 15. The presence of radiations in foods and plants is an concern as this leads to contamination of meals 16-18. Humans are exposed to uranium mainly through the soil-crop system because it easily becomes a part of the food chain. So, this review is compiled to focus on biogeochemical behavior of U in soil-plant system and various impacts of this heavy metal.
Sources of Uranium
Natural U comprises three isotopes: U238, U235, and U234 19. It contains about 99.283% of U238 by weight and rest U235 and U234 20. U238 has a half-life of 4.5×109 years and is an exceptionally long-lived isotope. Uranium is a radioactive element found in all types of soils, rocks, and water sources 21. Uranium comes from both natural and anthropogenic sources (Figure 1). Weathering of the rocks and volcanic eruption is considered the primary sources of natural U in soil 22,23. Other sources of Uranium includes mining, extracting and purifying ore, coal ash generation, phosphate fertilizer production, and waste from nuclear power plants 24. Wang et al., 2019 concluded that mining is the primary anthropogenic source of U contamination in soil and water 25. With ever exploding global population increase, this anthropogenic content of U will increase in the future due to higher demand for minerals, electricity, and food 8,26-28.
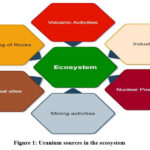 |
Figure 1: Uranium sources in the ecosystem |
Distribution and sources of Uranium in soil
Uranium concentration and radiation strength in the soil of different countries varies highly, as shown in Table 1 29-31. Highest concentration and radiation of Uranium in the soil was found in Portugal (25.10 mg/kg and 311.24 Bq/kg respectively). The average amount of uranium in the earth’s crust is about 2.82 mg kg-1 32,33. U can also leach or accumulate in specific soil profile horizons during the weathering process. Acid leaching is considered a significant factor in the distribution and mobility of Uranium in the soil profile 34. Sequestration and reduction are considered the primary factors for the high level of Uranium in soil rich in organic matter 35,36. U is retained in tropical environments in red soils due to its affinity for iron minerals 34,37. The source of contamination of Uranium varies greatly. A certain amount of Uranium is found in the coals 38-45, mining, extraction, and disposal of U-containing products or their by-products are considered the anthropogenic sources of U contamination of soils 46,47. Phosphorous fertilizers made from natural rocks in agriculture are another potential source of U enrichment in soils 48. The average value of U in such fertilizers is 100 times higher than in soils 49,50. The pollutant’s source and the intended use of the contaminated soil determine the concentration levels that are considered harmful. mobilization and transportation of water in vertical directions and at the surface depend on contaminated soil 51. Irrigation with U-contaminated water has also increased natural uranium in agricultural soil 52,53.
Factors affecting Uptake of Uranium by plants
The migration, uptake, and accumulation of minerals from the soil to the plant is a complex process involving runoff, capillary rise, leaching, sorption, and root uptake. The availability and uptake of essential macro and micronutrients like nitrogen, potassium, and zinc by plants influence the uptake of non-essential components. The term “bio-availability” refers to a chemical element’s tendency to adhere to or move across an organism’s cell surface 54; hence it determines how much concentration of essential and non-essential elements will be taken up by plants. Uranium uptake by plants is generally confined to the dissolved fraction in the soil, suggesting there might be lesser availability of uranium to plants. The uranyl ions are the only plant soluble and available fraction of U. Plants receive all macro and micronutrients from the soil through the movements of ions from the soil solution to the roots, including the uranyl ion. The translocation of U and other radionuclides is influenced by soil factors such as soil characteristics, climatic conditions, plant type, plant part concerned, the physicochemical form of the elements, and the presence of other elements influences the transfer factor values as well 55,56.
Concentration of Uranium in Soil
For uptake and accumulation of Uranium by the plant, it must be available in the soluble fraction of the soil; hence, the concentration of all elements, including the radionuclides and the intake of heavy metals inside the plant, is directly proportional to the concentration in the soil solution 57. The potential risk for uranium uptake and intake from different sources is higher for individuals who consume food grown in areas having soil with high concentrations of uranium because of its greater availability and absorption by plants 58 .
Soil pH
The availability and solubility of minerals and metals, including radionuclides in soils, depends on the soil’s pH. Different studies have concluded that the mobility and bioavailability during interaction with different soils are affected by pH 59. Heavy metal cations at neutral pH are strongly bounded to the soil minerals and hence are not bio-available. Since Uranium forms strong insoluble compounds, therefore it has low biological mobility at high pH, however, at low pH increases in heavy metal adsorption and hence increase in the concentration in plant parts are observed 61-63. Therefore, due to high metal bioavailability in highly acidic soil, metal toxicities are often observed in plants growing in such soil 64. Soil pH of less than 5.5 is required to convert U to its most plant available form in soil 61, as some ions in soils get adsorbed on oxides at low soil pH. So, the solubility of these cations and anions can be decreased by dissolving the Fe-, Mn-, and Al-oxides, which releases bound or adsorbed metals into the soil solution 62,64.
Organic matter in the Soil
The mobility of Uranium depends on organic components present in soil 65. Abdel-Haleem et al. (1997) found that organic wastes (biosolids) and municipal solid waste addition to soil increased the absorption of U in corn and sesame 66.
Uranium speciation
The mobilization and the solubility of uranium in both biotic and abiotic systems are a very complex process influenced by the uranium species present 67. The soil properties, especially pH and soil type, greatly influence U speciation 61,68,69 and are considered the key factors altering U uptake by plants. U(VI) is the most mobile and soluble form of U in soil70. U (VI)is present in solution mainly as UO22+ and soluble carbonate complexes 71,72. U (VI) exists primarily in hydrolyzed forms at a pH range of 4 – 7.5.73.
Soil type
The uptake of Uranium in the soil-plant system is not only confined to the bio-availability, but the several soil characteristics also help and influence the uranium sorption, subsequent desorption of metals, and uptake in plants 74. Ramaswami et al. (2001) discovered that the efficiency of uranium extraction in hydroponics and two different soils (sandy-loam and organic-rich soil) reduced sharply from hydroponics to sandy and then organic soil, indicating that soil organic matter sequest uranium, making it largely unavailable for plant uptake 75.
Soil Chelates
The presence of Chelates increases the accumulation of Uranium 63. The Chelates available in the soil bind metals and acidify the soil solution, increasing the bioavailability that aids in the translocation of metals from root to shoot 76. Citric acid has a high rate of environmental degradation, making it the most eco-friendly chelate for phytoextraction 62.
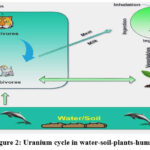 |
Figure 2: Uranium cycle in water-soil-plants-humans |
Table 1: Comparison of Uranium concentration and radiation in soils of different countries
|
Sr. No. |
Country |
U238 mg/kg |
U238 Bq/kg |
References |
|
1 |
India |
11 |
54 |
77 |
|
2 |
England |
2.6 |
32.24 |
77 |
|
3 |
Malaysia |
9.43 |
117 |
78 |
|
4 |
Greece |
2.25 |
28 |
79 |
|
5 |
Turkey |
1.11 |
13.8 |
80 |
|
6 |
Pakistan |
3.62 |
45 |
81 |
|
7 |
Germany |
1.90 |
23.56 |
82 |
|
8 |
Portugal |
25.10 |
311.24 |
83 |
|
9 |
Spain |
13.5 |
167.4 |
84 |
|
10 |
Japan |
1.74 |
21.57 |
85 |
|
11 |
USA |
3.50 |
43.4 |
86 |
|
12 |
Chile |
0.79 |
9.79 |
87 |
|
13 |
Canada |
1.20 |
14.88 |
77 |
|
14 |
China |
3.13 |
38.81 |
88 |
|
15 |
Europe |
2.46 |
30.50 |
89 |
|
16 |
World |
2.82 |
35 |
33 |
Traslocation of Uranium in Plants
U(VI) salts as UO2 2+and carbonate complexes are the most mobile form of Uranium 71, 90 while Other forms are less bioavailable and hence remain confined to soil particles. The Mycorrhiza fungi (Glomus genus), because of their high binding capacity for heavy metals, including Uranium, enhance their immobilization and significantly increase their plant uptake 55,91,92,93. Fungal mycelium via fungal tissues helps transport uranyl cations to roots 91-94 This has been proven by an experimental study done on Medicago trunctula cv. Jemalong plants in comparing treatments with and without the presence of the mycorrhizal fungus Glomus intraradices 55. It was also concluded that experimental plants infected with the fungus have higher U uptake by roots. In the inoculated plants, the concentrations of uranium in stems were higher, indicating that mycorrhizal root colonization increased U uptake. Organic acids also stimulate the phytoextraction of U 62. U uptake is also likely related to plant iron content 95. Gunther et al. (2003) showed that Uranium is most likely bound to phosphoryl groups as uranyl (VI) phosphate 96. Various growth anomalies and the highest concentration of uranium in the stems of Capsicum annum Cucumis in experiments conducted on Capsicum annuum and Cucumis sativus plants treated with uranium nitrate salts 97. Plants can absorb these elements in water-soluble forms, which are distributed from roots to aerial parts. While U contents greater than 3 mg/kg in tissues dry mass has been observed in some plants like Uncinia leptostachya and Coprosma Arborea (Mamangi). 98,99
Root to shoot Parts translocation
Accumulating uranium in root parts via soil or water takes place through penetration into the shoots and leaves (Table 2 and Table 3). In various plant species, such as sunflowers 100, Pisum sativum L. 101, Nicotiana tabacum L. 102, maize, wheat and pea 72, carlina corymbosa 103, sweet potato 104, mustard 105 U in roots and stem parts has been observed. Its translocation in the upper parts of the plants, including Sesbania rostrata 106, Water lily 107, Bidens pilosa L 108 depends on the potential of U in soil and gene expression in plants 109- 111, . Generally, the translocation of U from roots to the upper part of plants depends on three mechanisms, i.e., sequestration into root cells, symplastic transport among the central part of the plants, and its release through xylem 112-114. Uranium is transported after the formation of U chelates, i.e., UO2-citrate- and UO2-lactate in xylem tissues114 In the symplastic process, U ions from roots transfer to xylem vessels, probably due to transpiration 115,116. The selective permeability of the cell plasma membrane also regulates the Uranium transport through Membrane transport proteins 116,117.
Uranium toxicity
Uranium toxicity to plants
Uranium is toxic to plants, and factors like organic acids (citrate, tartrate, and oxalate), phosphate content, and polyamines affect its bioavailability 118-120. The cultivation substrate and its nature also influence the amount, distribution, movement, and toxicity level of Uranium in tobacco plants 102. At various pH levels, U has a considerable impact on Arabidopsis thaliana’s photosynthesis pathway 118. Since uranium is a toxic element for plants, it hinders the various physiological and biochemical processes like seed germination and photosynthesis. In addition, it causes damage to the structure of DNA and blocks the process of mitosis. Plant toxicity is mainly due to their environmental conditions, uranium concentration, and types of species 72, 108, 121.
Table 2: Uranium uptake by different plant species during pot experiment
|
Plants |
Roots (mg/kg) |
Shoot (mg/kg) |
Uranium Treatment (mg/kg) |
References |
|
Sesbania rostrata |
20.61 |
23.74 |
80 |
106 |
|
Juncus bufonius |
39.9 |
2.5 |
135 |
103 |
|
Maize |
32.01 |
3.50 |
50 |
72 |
|
Sunflower |
136 |
4.08 |
82 |
100 |
|
Italian Ryegrass |
800 |
290 |
150 |
122 |
|
Wild ramie |
721.46 |
35.88 |
7.98 |
123 |
|
Zebrina |
20.91 |
1.23 |
15 |
124 |
|
Juncus squarrosus |
227 |
1.1 |
250 |
103 |
|
Mustard |
7145 |
380 |
47.74 |
105 |
|
Carlina corymbosa |
134 |
0.9 |
149 |
103 |
|
Macleaya cordata |
36.8 |
12.5 |
18 |
125 |
Effect on Germination
Germination under U stresses vary from plant to plant as each plant tends to tolerate some level of U concentration. The results obtained from the germination of cleome amblyocarpa Barr. & murb seeds showed increase in the germination upto 200 ppm, and after that decrease at higher concentrations was noticed (250 ppm and 300 ppm) 126. In case of three vegetables (tomato, spinach, and cabbage) germination was inhibited at 320 ppm, whereas in cucumber, it was inhibited at 1280 ppm 127. Similar types of results were observed in cynodon dactylon (Bermuda) [6] and aristida purpura (purple Threeawn)128. U concentration lower than 100 ppm did not affect the germination of maize seeds but at higher concentrations a reduction to 80% and 63% in germination percentage has been observed at 500 ppm and 1000 ppm, respectively. This might be because at a lower concentration of Uranium, some enzymes promote seedling growth, or the net photosynthetic rate increases and thus enhances seed germination 126. When the U concentration reaches the maximum limit of tolerance power of the seed, its metabolic activities get disturbed and damage the DNA structure of plant cells, thus decreasing the rate of seed germination 7, 127,132.
Table 3: Uptake of Uranium under hydroponics conditions by different plant species
|
Plant species |
Roots(mg/kg) |
Shoot Parts (mg/kg) |
Hydroponics (umol/l) |
References |
|
Sweet potato |
2216 |
6.67 |
25 |
104 |
|
Purple sweet potato |
5712 |
3.48 |
25 |
104 |
|
Water lily |
1538 |
3446 |
55 |
107 |
|
Nicotiana tabacum L. |
82000 |
357 |
500 |
102 |
|
Wheat |
12000 |
28 |
100 |
129 |
|
Pea |
44000 |
21 |
100 |
129 |
|
Maize |
29737 |
6 |
100 |
129 |
|
Indian Mustard |
36541 |
122 |
100 |
129 |
|
Arabidopsis Halleri |
3500 |
170 |
– |
130 |
|
Arabidopsis Thaliana |
50352 |
15 |
50 |
131 |
|
Bidens pilosa L. |
728 |
809 |
1000 |
108 |
Uranium treatment on the seeds has an adverse effect on mitotic cell division. Furthermore, it leads to chromosomal cell defects 133. According to a study conducted on the Vicia faba a decrease in the mitotic index has been observed on the root tip cell 134 . It was found that uranium adversely affected the germination rate and seedling growth, and the level of toxicity depends upon the physiological state and selective permeation of different metal ions through tissues surrounding the embryo and hence determines the toxicity. Seedling growth is severely inhibited at a much lower concentration of heavy metal. The early visible symptoms of toxicity are disturbances of germination and change in leaf color, and germination percentage is negatively correlated with uranium concentration 135 .
Effect on Photosynthesis
Heavy metal stress is already known to affect photosynthesis, resulting in decreased plant growth, delayed plant development, and sometimes plant death 136 , 116. Reducing chlorophyll content is one of the harmful effects of exposing plants to various metals 137. Reductions in the chlorophyll a and chlorophyll b content due to the toxic effect of uranium has also been observed in different plant species such as Bidens pilosa L. 108 , Arabidopsis thaliana or Thale cress 131 , Pisum sativum L. is also called garden pea 143, Triticum aestivum L. 139 , Leptochloa fusca L. 140 , Nymphaea tetragona Georgi 107, Pisum sativum L. 101 and Green broad bean 138 .
According to Shtangeeva and Ayrault, U treatment increased light’s coefficient of reflection (CR) at spectral channel 0.38-0.63m, indicating a low chlorophyll content in the plant 141 . This decrease in chlorophyll biosynthesis is because of the replacement of Mg2+ ions by (UO2)2+ 142 . Uranium toxicity may disrupt the first step in glycolysis by replacing magnesium with uranyl in the enzyme 143 . Jagetiya and Purohit (2006) have also observed a gradual and contrasting reduction in the chlorophyll a, b and total chlorophyll content with increasing uranium concentration 144 .
Effect on Plant Physiology
Since uranium accumulates in plant roots, shoots adversely affect plant physiological parameters. The root and shoot length decreased significantly in Arabidopsis thaliana at 50uM U 145, duckweed at 50 uM solution of uranium 146 , and broad bean at 25uM U 147 . A decrease in root shoot fresh weight of Phaseolus vulgaris at 1000 uM U has been observed 143 and in the weight of fresh leaves at 100 uM U in Thale cress 148. In Ryegrass, maize, radish, and cabbage, the length of root and dry mass and stem height decreased significantly at 150mg/kg, 500mg/kg, and 2560 mg/kg uranium, respectively 72,127,122.
Uranium toxicity in human
The natural uranium isotopes 234U, 235U, 238U) decay to emit alpha, beta, and gamma rays, presenting both chemotoxicity and radiotoxicity effects in humans 149 . Uranium can enter the body in three routes: inhalation, ingestion, and absorption through intact or damaged skin 150. Various anthropogenic activities like nuclear power plants, military practices led to the formation of suspended uranium in air. Thus it can easily inhaled by humans and its radiotoxicity directly affect at the cellular, subcellular and protein levels, similarly it also affects kidney 151. Human beings also exposed through environmental uranium from ingesting water or food in natural uranium-contaminated areas 152 . Hence consumption of food, especially vegetables, fruits, cereals, and table salt, is the primary source of Uranium in the human body 153 , 154 . Cothern and Lappenbusch (1983) conducted study and found that food contributes 15 percent of the ingested U, while on other hand drinking water contributes 85% of Uranium 155 . The solubility of the uranium from consumed food affects the gastrointestinal absorption of uranium, with a variation in absorption rate from 0.1-31% 156-159. Uranium entry through contaminated water finds its way directly into the human bloodstream and has a negative impact on human health. The daily uranium intake is estimated to be 1-2 µg and 1.5 µg from food and water, respectively 160 . The human body contains an average of 56 µg uranium, attributing 32 µg (56%) to the skeleton, 11 g to muscle tissue, 9 g in fat, 2 µg in blood, and less than 1 g in the body organs like kidney, lungs, etc. 161 . Abnormalities in the gene, gulf war syndrome, infertility, and neurotoxic effects, occur due to uranium in the human body 162 . Accumulating uranium causes lung, bone, and thyroid cancer in humans. Sometimes higher intakes result in acute renal failure and even death 163-165. Its concentration builds up in the human body’s organs and tissues, posing various health risks 166,167 . It causes chronic problems with the liver, kidneys, and bones 168-170. The absorption of uranium into blood as an uranyl anions which further complexed with proteins (such as transferrin, albumin, or bicarbonate anions, etc 171 . The two main target organs of U are kidney and bone. More than 80 percent of the uranium is eliminated from the blood compartment via urethral excretion. The main target of uranium in human cells is mitochondria which ultimately leads to apoptosis. The geological origin of soils, groundwater, and flora’s living area has a significant impact on U transfer (Figure. 3) to the human food chain 172.
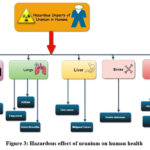 |
Figure 3: Hazardous effect of uranium on human health |
Conclusion
Natural radioactive minerals uranium is found int in rocks, soils, and water. But with increased industrialization and population explosion, its environmental concentration is rising. Although Uranium is not necessary for plants, it is taken up by the plants along with specific essential metals like Zn, Ni, Ca, and Cu. At a lower concentration, it does not pose any harm to plants. However, if the concentration of uranium reaches its threshold level, in that case, it causes direct toxicity causing damage to the plant by disturbing the cell structure (due to the production of reactive oxygen species causing oxidative stress), and it also inhibits several cytoplasmic enzymes. The uptake, retention, movement, and distribution profile of radionuclides in plants is strongly affected by the soil properties like pH, organic matter contents, soil characteristics, climatic conditions and, also by plant type, plant parts, the physicochemical form of the U and soil amendments such as fertilizer and chelate application. Transfer Factor (TF) estimates the quantity of uranium taken up by plants from the substrate. The U taken up by plants is translocated to the others parts of the plant. However, the concentration of U in different parts of plants follows the trend of roots>shoots>leaves. It adversely affects the germination of seeds and early seedling growth in plants. Uranium treatments in plants negatively affected the mitotic division and caused chromosomal abnormalities in seeds. Plant yield, shoot growth, root growth, and dry matter of plants are significantly reduced due to uranium uptake. So, it can be concluded that uranium absorption by plants from contaminated soil directly impacts plant development and yield and finally leads to the food crisis. Consumption of contaminated plant parts is the primary source of Uranium entry into the human food chain, and it represents a high potential risk to human health due chemical toxicity of Uranium.
Acknowledgement
Authors highly thankful to Department of Environmental Science, Maharshi Dayanand University, Rohtak, Haryana-124001.
Conflict of Interest
Authors declare that there is no conflict of interest.
Funding Sources
There are no funding Sources.
References
- Monreal MJ, Diaconescu PL. The riches of uranium. Nature chemistry. 2010 May;2(5):424-.
- Yang R, Liu W. Nitrate contamination of groundwater in an agroecosystem in Zhangye Oasis, Northwest China. Environmental Earth Sciences. 2010 Jul;61(1):123-9. https://doi.org/10.1007/s12665-009-0327-7
- UNSCEAR. Sources and Effects of Ionizing Radiation:Annex B-epidemiological Studies of Cancer Risk Due to Low-dose-rate Radiation from Environmental Sources. United Nations Publication, New York.2018.
- Duhan SS, Khyalia P, Laura JS. A comprehensive analysis of health risk due to natural outdoor gamma radiation in southeast Haryana, India. Current Science (00113891). 2022 Jul 25;123(2). http;//doi.org/10.18520/cs/v123/i2/169-176
- Khyalia P, Laura JS, Khosla B, Sahoo SK, Tiwari SN, Nandal M. Analysis of effective equivalent dose to the organs and cancer risk assessment due to natural outdoor gamma radiation in Eastern Thar Desert, India. International Journal of Environmental Analytical Chemistry. 2022 Oct 10:1-13. https://doi.org/10.1080/03067319.2022.2130692
- Nie X, Ding D, Li G, Gao B, Wu Y, Hu N, Liu Y. Soil radionuclide contamination and radionuclide accumulation characteristics of competitive plants in a uranium tailings repository in South China. Research of Environmental Sciences. 2010;23(6):719-25.
- Gao N, Huang Z, Liu H, Hou J, Liu X. Advances on the toxicity of uranium to different organisms. Chemosphere. 2019 Dec 1;237:124548. https://doi.org/10.1016/j.chemosphere.2019.124548
- Vanhoudt N, Vandenhove H, Horemans N, Wannijn J, Bujanic A, Vangronsveld J, Cuypers A. Study of oxidative stress related responses induced in Arabidopsis thaliana following mixed exposure to uranium and cadmium. Plant physiology and biochemistry. 2010 Oct 1;48(10-11):879-86. https://doi.org/10.1016/j.plaphy.2010.08.005
- WHO. Guidelines for drinking-water quality. 4th ed. Geneva, 2011.
- Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P. Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Exposure and Health. 2016 Sep;8(3):331-48. https://doi.org/10.1007/s12403-016-0193-y
- Li P, Tian R, Xue C, Wu J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environmental Science and Pollution Research. 2017 May;24(15):13224-34. https:// doi. org/ 10. 1007/ s11356- 017- 8753-7
- Li P, He S, He X, Tian R. Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a paper wastewater irrigation area, northwest China. Exposure and Health. 2018 Dec;10(4):241-258. https:// doi.rg/ 10. 1007/ s12403- 17- 0258-6
- Ren X, Li P, He X, Su F, Elumalai V. Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Archives of environmental contamination and toxicology. 2021 Jan;80(1):74-91. https://doi.org/10.1007/s00244-020-00772-5
- Punia A, Bharti R, Kumar P. Hydrogeochemical Processes Governing Uranium Mobility: Inferences from the Anthropogenically Disturbed, Semi-arid Region of India. Archives of Environmental Contamination and Toxicology. 2021 Oct;81(3):386-96. https://doi.org/10.1007/s00244-021-00879-3
- Chen J, Qiu Z, Gao B. Morphological distribution characteristics and bioavailability of radionuclide uranium of farmland soil in a uranium deposit area. Fresenius Environmental Bulletin. 2018;27(4):1979-88.
- Canbazoglu C, Ilter S, Sahin-Bal S, Karatepe S, Dogru M. A preliminary study on radioactivity concentrations and dose assessment of some anticarcinogenic medicinal plants used in Turkey. Fresenius Environmental Bulletin. 2018 Jan 1;27(2):793-798.
- Ajayi OS, Fatile EO, Dike CG. Radiological toxicity of some fish and meat tissues consumed in southwestern Nigeria. Human and Ecological Risk Assessment: An International Journal. 2018 Jul 4;24(5):1151-9. https://doi.org/10.1080/ 10807039.2017.1408004.
- Khalil EI, Anwar R, Fayez-Hassan M. 226 Ra, 232 Th, and 40 K activity concentration in foodstuffs consumed in Egypt. Arab Journal of Nuclear Sciences and Applications (Online). 2018;51(1):46-56.
- Strumińska-Parulska D, Olszewski G, Moniakowska A, Zhang J, Falandysz J. Bolete mushroom Boletus bainiugan from Yunnan as a reflection of the geographical distribution of 210Po, 210Pb and uranium (234U, 235U, 238U) radionuclides, their intake rates and effective exposure doses. Chemosphere. 2020 Aug 1;253:126585. https://doi.org/10.1016/j.chemosphere.2020.126585
- Awad HA, Zakaly HM, Nastavkin AV, El Tohamy AM, El-Taher A. Radioactive mineralizations on granitic rocks and silica veins on shear zone of El-Missikat area, Central Eastern Desert, Egypt. Applied Radiation and Isotopes. 2021 Feb 1;168:109493. https://doi.org/10.1016/j.apradiso.2020.109493
- Gavrilescu M, Pavel LV, Cretescu I. Characterization and remediation of soils contaminated with uranium. Journal of hazardous materials. 2009 Apr 30;163(2-3):475-510. https://doi.org/10.1016/j.jhazmat.2008.07.103
- Carling GT, Rupper SB, Fernandez DP, Tingey DG, Harrison CB. Effect of atmospheric deposition and weathering on trace element concentrations in glacial meltwater at Grand Teton National Park, Wyoming, USA. Arctic, Antarctic, and Alpine Research. 2017 Aug 1;49(3):427-40. https://doi.org/10.1657/AAAR0016.071
- Luo, J. C., Hu, R. Z., Fayek, M., Bi, X. W., Shi, S. H., & Chen, Y. W. (2017). Newly discovered uranium mineralization at~ 2.0 Ma in the Menggongjie granite-hosted uranium deposit, South China. Journal of Asian Earth Sciences, 137, 241-249. https://doi.org/10.1016/j.jseaes.2017.01.021
- Sethy NK, Jha VN, Sutar AK, Rath P, Sahoo SK, Ravi PM, Tripathi RM. Assessment of naturally occurring radioactive materials in the surface soil of uranium mining area of Jharkhand, India. Journal of Geochemical Exploration. 2014 Jul 1;142:29-35. https://doi.org/10.1016/j.gexplo.2013.11.009
- Wang Z, Qin H, Wang J. Accumulation of uranium and heavy metals in the soil–plant system in Xiazhuang uranium ore field, Guangdong Province, China. Environmental geochemistry and health. 2019 Dec;41(6):2413-23. https://doi.org/10.1007/s10653-019-00286-7
- Hedges MM. Uranium exploration and production: a review of innovation. Applied Earth Science. 2008 Jun 1;117(2):51-4. https://doi.org/10.1179/174327508X324798
- Mitchell N, Pérez-Sánchez D, Thorne MC. A review of the behaviour of U-238 series radionuclides in soils and plants. Journal of Radiological Protection. 2013 Apr 23;33(2):R17.
- Paithankar JG, Ghodke TS, Patil RK. Insight into the evolutionary profile of radio-resistance among insects having intrinsically evolved defence against radiation toxicity. International Journal of Radiation Biology. 2022 Jun 3;98(6):1012-24. https://doi.org/10.1080/ 09553002.2020.1859153.
- Kabata PA, Pendias H. Trace Elements in the soil and plants CRC Press. Boca Raton FL. 1984.
- Pais I, Jones Jr JB. The handbook of trace elements. Crc Press; 1997 Apr 24.
- Sahoo SK. Measurement of uranium and its isotopes at trace levels in environmental samples using mass spectrometry. Indian Journal of Physics. 2009 Jun;83(6):787-97. https://doi.org/10.1007/s12648-009-0046-7
- Meinrath A, Schneider P, Meinrath G. Uranium ores and depleted uranium in the environment, with a reference to uranium in the biosphere from the Erzgebirge/Sachsen, Germany. Journal of Environmental Radioactivity. 2003 Jan 1;64(2-3):175-93. https://doi.org/10.1016/S0265-931X(02)00048-6
- UNSCEAR. Sources and effects of Ionizing Radiation, Report to the General Assembly, Vol. I. (New York : United Nations), 2000.
- Evans CV, Morton LS, Harbottle G. Pedologic assessment of radionuclide distributions: use of a radio‐pedogenic index. Soil Science Society of America Journal. 1997 Sep;61(5):1440-9. https://doi.org/10.2136/sssaj1997.03615995006100050023x
- Yoshida S, Muramatsu Y, Tagami K, Uchida S. Concentrations of lanthanide elements, Th, and U in 77 Japanese surface soils. Environment International. 1998 Apr 1;24(3):275-86. https://doi.org/10.1016/S0160-4120(98)00006-3
- Fellows RJ, Ainsworth CC, Driver CJ, Cataldo DA. Dynamics and transformations of radionuclides in soils and ecosystem health. Soil chemistry and ecosystem health. 1998 Jan 1;52:85-132. https://doi.org/10.2136/sssaspecpub52.c4
- Tipping E. Hydrochemical modelling of the retention and transport of metallic radionuclides in the soils of an upland catchment. Environmental Pollution. 1996 Jan 1;94(2):105-16. https://doi.org/10.1016/S0269-7491(96)00086-3
- Chen L, Liu J, Zhang W, Zhou J, Luo D, Li Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: a review. Journal of Hazardous Materials. 2021 Jul 5;413:125319. https://doi.org/10.1016/j.jhazmat.2021.125319
- Shao L, Jones T, Gayer R, Dai S, Li S, Jiang Y, Zhang P. Petrology and geochemistry of the high-sulphur coals from the Upper Permian carbonate coal measures in the Heshan Coalfield, southern China. International Journal of Coal Geology. 2003 Jun 1;55(1):1-26. https://doi.org/10.1016/s0166-5162(03)00031-4.
- Silva L F O, Oliveira M L S, Da Boit K M, Finkelman R B. Characterization of Santa Catarina (Brazil) coal with respect to human health and environmental concerns. Environmental Geochemistry and Health. 2009, 31(4), 475-485.. https://doi.org/10.1007/s10653- 008-9200-y.
- Ndhlalose M, Malumbazo N, Wagner N. Coal quality and uranium distribution in Springbok Flats Coalfield samples. Journal of the Southern African Institute of Mining and Metallurgy. 2015 Dec;115(12):1167-74. https://doi.org/10.17159/2411-9717/2015/v115n12a4.
- Qin S, Lu Q, Gao K, Bo P, Wu S. Geochemistry of elements associated with Late Permian coal in the Zhongliangshan mine, Chongqing, Southwest China. Energy Exploration & Exploitation. 2018 Nov;36(6):1655-73. https://doi.org/10.1177/ 0144598718768980.
- Ozden B, Guler E, Vaasma T, Horvath M, Kiisk M, Kovacs T. Enrichment of naturally occurring radionuclides and trace elements in Yatagan and Yenikoy coal-fired thermal power plants, Turkey. Journal of Environmental Radioactivity. 2018 Aug 1;188:100-7. https://doi. org/10.1016/j.jenvrad.2017.09.016
- Galhardi, J. A., de Mello, J. W., & Wilkinson, K. J. (2020). Environmental and health risk assessment of agricultural areas adjacent to uranium ore fields in Brazil. Environmental Geochemistry and Health, 42(11), 3965-3981. https://doi.org/10.1007/s10653-020-00659-3.
- Li X, Dai S, Nechaev VP, Graham IT, French D, Wang X, Zhao L, Zhao J. Mineral matter in the late permian C1 coal from Yunnan Province, China, with emphasis on its origins and modes of occurrence. Minerals. 2020 Dec 25;11(1):19. https://doi. org/10.3390/min11010019.
- Dreesen DR, Marple ML. Uptake of trace elements and radionuclides from uranium mill tailings by four-wing saltbush (Atriplex canescens) and alkali sacaton (Sporobolus airoides).[Radium 226; Uranium; Molybdenum; Selenium; Vanadium; Astatine]. Los Alamos National Lab.(LANL), Los Alamos, NM (United States); 1979 Jan 1.
- Dreesen DR, Williams JM, Marple ML, Gladney ES, Perrin DR. Mobility and bioavailability of uranium mill tailings contaminants. Environmental Science & Technology. 1982 Oct;16(10):702-9. https://doi.org/10.1021/es00104a013
- Dissanayake C, B Chandrajith R. Phosphate mineral fertilizers, trace metals and human health. Journal of the National Science Foundation of Sri Lanka. 2009, 37(3), 153-165.
- Lehr JR. Phosphate raw materials and fertilizers: Part I—A look ahead. The role of phosphorus in agriculture. 1980 Jan 1:81-120.
- Schnug E, Haneklaus S, Schnier C, Scholten LC. Issues of natural radioactivity in phosphates. Communications in Soil Science and Plant Analysis. 1996 Feb 1;27(3-4):829-41. https://doi.org/10.1080/00103629609369600
- Layton DW, Armstrong AQ. Methodological considerations for determining cleanup limits for uranium in treated and untreated soils. Soil and Sediment Contamination. 1994 Dec 1;3(4):319-48. https://doi.org/10.1080/15320389409383474
- Huynh NP, Le CH. Accumulation rates of natural radionuclides (40K, 210Pb, 226Ra, 238U, and 232Th) in topsoils due to long-term cultivations of water spinach (Ipomoea Aquatica Forssk.) and rice (Oryza Sativa L.) based on model assessments: A case study in Dong Nai province, Vietnam. Journal of Environmental Management. 2020 Oct 1;271:111001. https://doi.org/10.1016/j.jenvman.2020.111001
- Nguyen VT, Le BA, Huynh NP, Le CH. Levels of 226Ra in some paddy soils in the Mekong Delta region (Vietnam): current status and long-term assessment. Journal of Radioanalytical and Nuclear Chemistry. 2021 Aug;329(2):829-38. https://doi.org/10.1007/s10967-021-07870-1
- Markich SJ. Uranium speciation and bioavailability in aquatic systems: an overview. The Scientific World JOURNAL. 2002 Mar 15;2:707-29. https://doi.org/10.1100/tsw.2002.130
- Chen B, Roos P, Borggaard OK, Zhu YG, Jakobsen I. Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytologist. 2005 Feb;165(2):591-8. https://doi.org/10.1111/j.1469-8137.2004.01244.x
- Coughtrey PJ, Jackson D, Thorne MC. Radionuclide distribution and transport in terrestrial and aquatic ecosystems. A critical review of data. Volume 3. AA Balkema; 1983.
- Vandenhove H, Hees MV, Winckel SV. Feasibility of phytoextraction to clean up low-level uranium-contaminated soil. International Journal of Phytoremediation. 2001 Jul 1;3(3):301-20. https://doi.org/10.1080/15226510108500061
- EPA. Drinking water criteria document for uranium. Washington, DC: U.S. Environmental Protection Agency; PB86241049, 1985.
- Yu S, Ma J, Shi Y, Du Z, Zhao Y, Tuo X, Leng Y. Uranium (VI) adsorption on montmorillonite colloid. Journal of Radioanalytical and Nuclear Chemistry. 2020 May;324(2):541-9. https://doi.org/10.1007/s10967-020-07083-y
- Whicker FW, Schultz V. Radioecology: nuclear energy and the environment. Boca Raton, FL: CRC press; 1982 Jan 1.
- Ebbs SD, Brady DJ, Kochian LV. Role of uranium speciation in the uptake and translocation of uranium by plants. Journal of experimental botany. 1998 Jul 1;49(324):1183-90. https://doi.org/10.1093/jxb/49.324.1183
- Huang JW, Blaylock MJ, Kapulnik Y, Ensley BD. Phytoremediation of uranium-contaminated soils: role of organic acids in triggering uranium hyperaccumulation in plants. Environmental science & technology. 1998 Jul 1;32(13):2004-8. https://doi.org/10.1021/es971027u
- Shahandeh, H., & Hossner, L. R. (2002). Role of soil properties in phytoaccumulation of uranium. Water, Air, and Soil Pollution, 141(1), 165-180. https://doi.org/10.1023/A:1021346828490.
- Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Bio/technology. 1995 May;13(5):468-74. https://doi.org/10.1038/nbt0595-468
- Bednar AJ, Medina VF, Ulmer-Scholle DS, Frey BA, Johnson BL, Brostoff WN, Larson SL. Effects of organic matter on the distribution of uranium in soil and plant matrices. Chemosphere. 2007 Dec 1;70(2):237-47. https://doi.org/10.1016/j.chemosphere.2007.06.032
- Abdel-Haleem AS, Abdel-Sabour MF, El-Shershaby A, Walley El-Dine N. Assessment of uranium and thorium uptake in corn and sesame seeds due to organic waste application to sandy soil. Nuclear Science Journal-Taipei-. 1997;34:431-6.
- Merkel B, Planer-Friedrich B. Uranium in the Aquatic Environment: proceedings of the International Conference [on] Uranium Mining and Hydrogeology III and the International Mine Water Association Symposium, Freiberg, Germany, 15-21 September 2002: with 453 figures, 151 tables and a CD-ROM. Springer Science & Business Media; 2002.
- Langmuir D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochimica et Cosmochimica Acta. 1978 Jun 1;42(6):547-69. https://doi.org/10.1016/0016-7037(78)90001-7
- Mortvedt JJ. Plant and soil relationships of uranium and thorium decay series radionuclides—a review. Journal of Environmental Quality. 1994 Jul;23(4):643-50. https://doi.org/10.2134/jeq1994.00472425002300040004x
- Campbell MD, Biddle KT. Frontier Areas and Exploration Techniques: Frontier Uranium Exploration in the South-Central United States 1977;3-44.
- Grenthe I, Fuger J, Konings R, Lemire RJ, Muller A B, Nguyen-Trung C, Wanner J. The chemical thermodynamics of uranium (Vol. 1). Amsterdam:.Elsevier, New York 1992.
- Stojanović MD, Stevanović DR, Milojković JV, Grubišić MS, Ileš DA. Phytotoxic effect of the uranium on the growing up and development the plant of corn. Water, Air, & Soil Pollution. 2010 Jun;209(1):401-10. https://doi.org/10.1007/s11270-009-0208-4
- Meinrath G, Kato Y, Kimura T, Yoshida Z. Solid-aqueous phase equilibria of uranium (VI) under ambient conditions. Radiochimica Acta. 1996 Dec 1;75(3):159-68. https://doi.org/10.1524/ract.1996.75.3.159
- Vandenhove H, Van Hees M, Wouters K, Wannijn J. Can we predict uranium bioavailability based on soil parameters? Part 1: effect of soil parameters on soil solution uranium concentration. Environmental Pollution. 2007 Jan 1;145(2):587-95. https://doi.org/10.1016/j.envpol.2006.04.011
- Ramaswami A, Carr P, Burkhardt M. Plant-uptake of uranium: hydroponic and soil system studies. International Journal of Phytoremediation. 2001 Apr 1;3(2):189-201. https://doi.org/10.1080/15226510108500056
- Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environmental Science & Technology. 1997 Feb 27;31(3):860-5. https://doi.org/10.1021/es960552a
- Vodyanitskii YN. Chemical aspects of uranium behavior in soils: a review. Eurasian soil science. 2011 Aug;44(8):862-73. https://doi.org/10.1134/S1064229311080163
- Gabdo HT, Ramli AT, Saleh MA, Sanusi MS, Garba NN, Aliyu AS. Radiological hazard associated with natural radionuclide concentrations in the northern part of Pahang state Malaysia. Environmental Earth Sciences. 2015 May;73(10):6271-81. https://doi.org/10.1007/s12665-014-3850-0
- Papaefthymiou HV, Chourdakis G, Vakalas J. Natural radionuclides content and associated dose rates in fine-grained sediments from Patras-Rion sub-basins, Greece. Radiation protection dosimetry. 2011 Jan 1;143(1):117-24. https://doi.org/10.1093/rpd/ncq345
- Manisa K, Erdogan M, Usluer A, Cetinkaya H, Isik U, Sahin L, Zedef V. Assessment of natural radioactivity level of soil and water in the region of Çorlu (Turkey). Journal of Radioanalytical and Nuclear Chemistry. 2021 Sep;329(3):1213-21. https://doi.org/10.1007/s10967-021-07906-6
- Khan HM, Ismail M, Zia MA, Khan K. Measurement of radionuclides and absorbed dose rates in soil samples of Peshawar, Pakistan, using gamma ray spectrometry. Isotopes in environmental and health studies. 2012 Jun 1;48(2):295-301. https://doi.org/10.1080/10256016.2012.641963
- Utermann J, Fuchs M. Uranium in German soils. Loads and fate of fertilizer-derived uranium. 2008:33-47.
- Neiva AM, Carvalho PC, Antunes IM, Silva MM, Santos AC, Pinto MC, Cunha PP. Contaminated water, stream sediments and soils close to the abandoned Pinhal do Souto uranium mine, central Portugal. Journal of Geochemical Exploration. 2014 Jan 1;136:102-17. https://doi.org/10.1016/j.gexplo.2013.10.014
- Santos-Francés F, Pacheco EG, Martinez-Grana A, Rojo PA, Zarza CÁ, Sánchez AG. Concentration of uranium in the soils of the west of Spain. Environmental Pollution. 2018 May 1;236:1-1.
- Sahoo SK, Hosoda M, Kamagata S, Sorimachi A, Ishikawa T, Tokonami S, Uchida S. Thorium, uranium and rare earth elements concentration in weathered Japanese soil samples. Progress in Nuclear Science and Technology. 2011 Feb 15;1:416-9.
- Bern CR, Walton-Day K, Naftz DL. Improved enrichment factor calculations through principal component analysis: Examples from soils near breccia pipe uranium mines, Arizona, USA. Environmental pollution. 2019 May 1;248:90-100. https://doi.org/10.1016/j.envpol.2019.01.122
- Pinto MC, da Silva EF, Silva MM, Dinis PA. Estimated background values maps of uranium in Santiago Island topsoil and stream sediments. Procedia Earth and Planetary Science. 2014 Jan 1;8:23-7. https://doi.org/10.1016/j.proeps.2014.05.006
- Xu N, Wei FS, Ten EJ, Chen LQ. Evaluation of indigenous concentrations of uranium and thorium in soils of China. Communications in soil science and plant analysis. 1993 Sep 1;24(15-16):1795-803. https://doi.org/10.1080/00103629309368918
- Plant JA, Reeder S, Salminen R, Smith DB, Tarvainen T, De Vivo B, Petterson MG. The distribution of uranium over Europe: geological and environmental significance. Applied Earth Science. 2003 Dec 1;112(3):221-38. https://doi.org/10.1179/037174503225003152
- Duff MC, Amrhein C. Uranium (VI) adsorption on goethite and soil in carbonate solutions. Soil Science Society of America Journal. 1996 Sep;60(5):1393-400. https://doi.org/10.2136/sssaj1996.03615995006000050014x
- Rufyikiri G, Thiry Y, Wang L, Delvaux B, Declerck S. Uranium uptake and translocation by the arbuscular mycorrhizal fungus, Glomus intraradices, under root‐organ culture conditions. New Phytologist. 2002 Nov;156(2):275-81. https://doi.org/10.1046/j.1469-8137.2002.00520.x
- Rufyikiri G, Thiry Y, Declerck S. Contribution of hyphae and roots to uranium uptake and translocation by arbuscular mycorrhizal carrot roots under root-organ culture conditions. New Phytologist. 2003 May 1:391-9. : 10.1046/j.1469-8137.2003.00747.x
- Rufyikiri G, Declerck S, Thiry Y. Comparison of 233U and 33P uptake and translocation by the arbuscular mycorrhizal fungus Glomus intraradices in root organ culture conditions. Mycorrhiza. 2004 Jun;14(3):203-7.https://doi.org/10.1007/s00572-003-0258-1
- Weiersbye IM, Straker CJ, Przybylowicz WJ. Micro-PIXE mapping of elemental distribution in arbuscular mycorrhizal roots of the grass, Cynodon dactylon, from gold and uranium mine tailings. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 1999 Sep 2;158(1-4):335-43. https://doi.org/10.1016/S0168-583X(99)00467-X
- Rodrıguez PB, Tome FV, Lozano JC. About the assumption of linearity in soil-to-plant transfer factors for uranium and thorium isotopes and 226Ra. Science of the total environment. 2002 Feb 4;284(1-3):167-75. https://doi.org/10.1016/S0048-9697(01)00877-4
- Günther A, Bernhard G, Geipel G, Reich T, Rossberg A, Nitsche H. Uranium speciation in plants. Radiochimica Acta. 2003 Jun 1;91(6):319-28. https://doi.org/10.1524/ract.91.6.319.20022
- Ünak T, Yildirim Y, Tokucu G, Ünak G, Öcal J, Konyali D, Kiliç S. Study of the effect of uranium and thorium on the growing of pepper (Capsicum annuum var. longum) and cucumber (Cucumis sativus) plants. Journal of radioanalytical and nuclear chemistry. 2007 Sep 1;273(3):763-6. https://doi.org/10.1007/s10967-007-0944-0
- Peterson PJ. Unusual accumulations of elements by plants and animals. Science Progress (1933-). 1971 Dec 1:505-26.
- Whiting SN, Reeves RD, Richards D, Johnson MS, Cooke JA, Malaisse F, et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restoration Ecology. 2004 Mar;12(1):106-16. https://doi.org/10.1111/j.1061-2971.2004.00367.x
- Meng F, Jin D, Guo K, Larson SL, Ballard JH, Chen L, Arslan Z, et al. Influences of U sources and forms on its bioaccumulation in Indian Mustard and Sunflower. Water, Air, & Soil Pollution. 2018 Nov;229(11):1-1. https://doi.org/10.1007/s11270-018-4023-7
- Gupta DK, Vuković A, Semenishchev VS, Inouhe M, Walther C. Uranium accumulation and its phytotoxicity symptoms in Pisum sativum L. Environmental Science and Pollution Research. 2020 Jan;27(3):3513-22. https://doi.org/10.1007/s11356-019-07068-9
- Soudek P, Petrová Š, Buzek M, Lhotský O, Vaněk T. Uranium uptake in Nicotiana sp. under hydroponic conditions. Journal of Geochemical Exploration. 2014 Jul 1;142:130-7. https://doi.org/10.1016/j.gexplo.2013.10.001
- Favas PJ, Pratas J, Mitra S, Sarkar SK, Venkatachalam P. Biogeochemistry of uranium in the soil-plant and water-plant systems in an old uranium mine. Science of the total environment. 2016 Oct 15;568:350-68. https://doi.org/10.1016/j.scitotenv.2016.06.024
- Lai JL, Liu ZW, Li C, Luo XG. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. Journal of Hazardous Materials. 2021 May 5;409:124997. https://doi.org/10.1016/j.jhazmat.2020.124997
- Qi F, Zha Z, Du L, Feng X, Wang D, Zhang D, Fang Z, Ma L, Jin Y, Xia C. Impact of mixed low-molecular-weight organic acids on uranium accumulation and distribution in a variant of mustard (Brassica juncea var. tumida). Journal of Radioanalytical and Nuclear Chemistry. 2014 Oct;302(1):149-59. https://doi.org/10.1007/s10967-014-3279-7
- Ren CG, Kong CC, Wang SX, Xie ZH. Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere. 2019 Feb 1;217:773-9. https://doi.org/10.1016/j.chemosphere.2018.11.085
- Li C, Wang M, Luo X, Liang L, Han X, Lin X. Accumulation and effects of uranium on aquatic macrophyte Nymphaea tetragona Georgi: Potential application to phytoremediation and environmental monitoring. Journal of environmental radioactivity. 2019 Mar 1;198:43-9. https://doi.org/10.1016/j.jenvrad.2018.12.018
- Imran M, Hu S, Luo X, Cao Y, Samo N. Phytoremediation through Bidens pilosa L., a nonhazardous approach for uranium remediation of contaminated water. International journal of phytoremediation. 2019 Jul 3;21(8):752-9. https://doi.org/10.1080/15226514.2018.1556594
- Vanhoudt N, Vandenhove H, Horemans N, Wannijn J, Van Hees M, Vangronsveld J, Cuypers A. The combined effect of uranium and gamma radiation on biological responses and oxidative stress induced in Arabidopsis thaliana. Journal of environmental radioactivity. 2010 Nov 1;101(11):923-30. https://doi.org/10.1016/j.jenvrad.2010.06.008
- Doustaly F, Combes F, Fiévet JB, Berthet S, Hugouvieux V, Bastien O et al. Uranium perturbs signaling and iron uptake response in Arabidopsis thaliana roots. Metallomics. 2014 Apr;6(4):809-21. .https://doi.org/10.1039/c4mt00005f
- Wu R, Fan Y, Wu Y, Zhou S, Tang S, Feng X, Tan X, et al. Insights into mechanism on organic acids assisted translocation of uranium in Brassica juncea var. foliosa by EXAFS. Journal of environmental radioactivity. 2020 Jul 1;218:106254. https://doi.org/10.1016/j.jenvrad.2020.106254
- Sheoran V, Sheoran AS, Poonia P. Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: a review. Critical Reviews in Environmental Science and Technology. 2010 Dec 30;41(2):168-214. https://doi.org/10.1080/10643380902718418
- Mihalík J, Henner P, Frelon S, Camilleri V, Février L. Citrate assisted phytoextraction of uranium by sunflowers: Study of fluxes in soils and plants and resulting intra-planta distribution of Fe and U. Environmental and experimental botany. 2012 Apr 1;77:249-58. https://doi.org/10.1016/j.envexpbot.2011.11.024
- Pentyala VB, Eapen S. High efficiency phytoextraction of uranium using Vetiveria zizanioides L. Nash. International Journal of Phytoremediation. 2020 Sep 18;22(11):1137-46. https://doi.org/10.1080/15226514.2020.1741506
- Aranjuelo I, Doustaly F, Cela J, Porcel R, Müller M, Aroca R, Munné-Bosch S, Bourguignon J. Glutathione and transpiration as key factors conditioning oxidative stress in Arabidopsis thaliana exposed to uranium. Planta. 2014 Apr;239(4):817-30. https://doi.org/10.1007/s00425-013-2014-x
- Berthet S, Villiers F, Alban C, Serre NB, Martin‐Laffon J, Figuet S, Boisson AM, Bligny R, Kuntz M, Finazzi G, Ravanel S. Arabidopsis thaliana plants challenged with uranium reveal new insights into iron and phosphate homeostasis. new phytologist. 2018 Jan;217(2):657-70. https://doi.org/10.1111/nph.14865
- Rufyikiri G, Huysmans L, Wannijn J, Van Hees M, Leyval C, Jakobsen I. Arbuscular mycorrhizal fungi can decrease the uptake of uranium by subterranean clover grown at high levels of uranium in soil. Environmental Pollution. 2004 Aug 1;130(3):427-36. https://doi.org/10.1016/j.envpol.2003.12.021
- Saenen E, Horemans N, Vanhoudt N, Vandenhove H, Biermans G, Van Hees M, et al. The pH strongly influences the uranium-induced effects on the photosynthetic apparatus of Arabidopsis thaliana plants. Plant physiology and biochemistry. 2014 Sep 1;82:254-61. https://doi.org/10.1016/j.plaphy.2014. 06.012.
- Waseem A, Ullah H, Rauf MK, Ahmad I. Distribution of natural uranium in surface and groundwater resources: a review. Critical reviews in environmental science and technology. 2015 Nov 17;45(22):2391-423. https://doi.org/10.1080/10643389.2015.1025642.
- Croteau MN, Fuller CC, Cain DJ, Campbell KM, Aiken G. Biogeochemical controls of uranium bioavailability from the dissolved phase in natural freshwaters. Environmental science & technology. 2016 Aug 2;50(15):8120-7. https://doi.org/10.1021/acs.est.6b02406.
- Sachs S, Geipel G, Bok F, Oertel J, Fahmy K. Calorimetrically determined U (VI) toxicity in Brassica napus correlates with oxidoreductase activity and U (VI) speciation. Environmental Science & Technology. 2017 Sep 19;51(18):10843-9. https://doi.org/10.1021/acs.est.7b02564.
- Qi X, Hao X, Chen X, Xiao S, Chen S, Luo X, et al. Integrated phytoremediation system for uranium-contaminated soils by adding a plant growth promoting bacterial mixture and mowing grass. Journal of soils and sediments. 2019 Apr;19(4):1799-808. https://doi.org/10.1007/s11368-018-2182-1
- Wang WH, Luo XG, Liu L, Zhang Y, Zhao HZ. Ramie (Boehmeria nivea)’s uranium bioconcentration and tolerance attributes. Journal of Environmental Radioactivity. 2018 Apr 1;184:152-7. https://doi.org/10.1016/j.jenvrad.2018.01.016
- Chen L, Wang D, Long C, Cui ZX. Effect of biodegradable chelators on induced phytoextraction of uranium-and cadmium-contaminated soil by Zebrina pendula Schnizl. Scientific reports. 2019 Dec 24;9(1):1-9. https://doi.org/10.1038/s41598-019-56262-9.
- Hu N, Lang T, Ding D, Hu J, Li C, Zhang H, Li G. Enhancement of repeated applications of chelates on phytoremediation of uranium contaminated soil by Macleaya cordata. Journal of environmental radioactivity. 2019 Apr 1;199:58-65. https://doi.org/10.1016/j.jenvrad.2018.12.023
- Aicha B, Abdelhakim RY, Tayeb N, Nacer F, Lazreg B, Hanane H, Elhouda NN. Effect of uranium on seed germination of Cleome amblyocarpa Barr. & Murb. Plant Archives. 2019;19(2):3805-10.
- Hou J, Wang C, Zhou Y, Li S, Hayat T, Alsaedi A, Wang X. Effects of uranium stress on physiological and biochemical characteristics in seedlings of six common edible vegetables. Journal of Radioanalytical and Nuclear Chemistry. 2018 Jun;316(3):1001-10. https://doi.org/10.1007/s10967-018-5792-6
- Butler AD, Wynter M, Medina VF, Bednar AJ. Depleted uranium toxicity, accumulation, and uptake in Cynodon dactylon (Bermuda) and Aristida purpurea (purple Threeawn). Bulletin of environmental contamination and toxicology. 2016 Jun;96(6):714-9. https://doi.org/10.1007/s00128-016-1784-9
- Straczek A, Duquene L, Wegrzynek D, Chinea-Cano E, Wannijn J, Navez J, Vandenhove H. Differences in U root-to-shoot translocation between plant species explained by U distribution in roots. Journal of environmental radioactivity. 2010 Mar 1;101(3):258-66. https://doi.org/10.1016/j.jenvrad.2009.11.011
- Viehweger K, Geipel G. Uranium accumulation and tolerance in Arabidopsis halleri under native versus hydroponic conditions. Environmental and experimental botany. 2010 Sep 1;69(1):39-46. https://doi.org/10.1016/j.envexpbot.2010.03.001.
- Vanhoudt N, Horemans N, Biermans G, Saenen E, Wannijn J, Nauts R, Van Hees M, Vandenhove H. Uranium affects photosynthetic parameters in Arabidopsis thaliana. Environmental and experimental botany. 2014 Jan 1;97:22-9. https://doi.org/10.1016/j.envexpbot.2013.09.009
- Chen L, Yang JY, Wang D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. Journal of Cleaner Production. 2020 Aug 1;263:121491. https://doi.org/10.1016/j.jclepro.2020.121491
- Özdemir C, Ereeş FS, Sepet H, Bozdağ B, Yetişen K, Şen U, Çam S. Cytogenetic Effects of Uranium on Root Tip Cells of Fabaceae (Cicer arietinum L., Phaseolus vulgaris L., Vigna anguiculata L. and Phaseolus coccineus L.). Middle-East J. Sci. Res. 2012;11(6):791-5.
- Özdemir C, Ereeş FS, Çam S. CYTOGENETIC EFFECTS OF URANIUM ON ROOT TIP CELLS OF VICIA FABA. Botanica Lithuanica (1392-1665). 2008 Sep 1;14(3).
- Li W, Khan MA, Yamaguchi S, Kamiya Y. Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant growth regulation. 2005 May;46(1):45-50. https://doi.org/10.1007/s10725-005-6324-2
- Chen X, Wu G, Ma Q, Lai JL, Luo XG, Ji XH. Cytotoxic and genotoxic evaluation and the toxicological mechanism of uranium in Vicia faba root. Environmental and Experimental Botany. 2020 Nov 1;179:104227. https://doi.org/10.1016/j.envexpbot.2020.104227
- Ernst WH. Evolution and ecophysiology of metallophytes in Africa. InResults of Worldwide Ecological Studies. First Symposium AFW Schimper Foundation. 2000 (pp. 23-35). G. Hembach Verlag.
- Tawussi F, Walther C, Gupta DK. Does low uranium concentration generates phytotoxic symptoms in Pisum sativum L. in nutrient medium?. Environmental Science and Pollution Research. 2017 Oct;24(28):22741-51. https://doi.org/10.1007/s11356-017-0056-5
- Chen X, Tang Y, Zhou L, Chen M, Wang D. Accumulation and distribution of uranium in wheat seedling and the effects of uranium on the photosystem activities. Acta Botanica Boreali-Occidentalia Sinica. 2012;32(12):2457-63.
- Ahsan MT, Najam-ul-Haq M, Idrees M, Ullah I, Afzal M. Bacterial endophytes enhance phytostabilization in soils contaminated with uranium and lead. International journal of phytoremediation. 2017 Oct 3;19(10):937-46. https://doi.org/10.1080/15226514.2017.1303813
- Shtangeeva I, Ayrault S. Phytoextraction of thorium from soil and water media. Water, air, and soil pollution. 2004 May;154(1):19-35. https://doi.org/10.1023/B:WATE.0000022927.15629.04
- Hafez M, Ramadan Y. Treatment of radioactive and industrial liquid wastes by Eichornia crassipes. Journal of radioanalytical and nuclear chemistry. 2002 Jun 17;252(3):537-40. https://doi.org/10.1023/a:1015806921332
- Vandenhove H, Cuypers A, Van Hees M, Koppen G, Wannijn J. Oxidative stress reactions induced in beans (Phaseolus vulgaris) following exposure to uranium. Plant Physiology and Biochemistry. 2006 Nov 1;44(11-12):795-805. https://doi.org/10.1016/j.plaphy.2006.10.013
- Jagetiya BL, Purohit P. Effects of various concentrations of uranium tailings on certain growth and biochemical parameters in sunflower. Biologia. 2006 Feb;61(1):103-7. https://doi.org/10.2478/s11756-006-0015-y
- Misson J, Henner P, Morello M, Floriani M, Wu TD, Guerquin-Kern JL, Février L. Use of phosphate to avoid uranium toxicity in Arabidopsis thaliana leads to alterations of morphological and physiological responses regulated by phosphate availability. Environmental and experimental botany. 2009 Dec 1;67(2):353-62. https://doi.org/10.1016/j.envexpbot.2009.09.001
- Mkandawire M, Vogel K, Taubert B, Dudel EG. Phosphate regulates uranium (VI) toxicity to Lemna gibba L. G3. Environmental Toxicology: An International Journal. 2007 Feb;22(1):9-16. https://doi.org/10.1002/tox.20228
- Liu Z, Lai J, Li J, Ding F, Zhang Y, Luo X. Toxic mechanism of uranium on photosynthetic characteristics and respiratory metabolism of Vicia faba L. Nongye Huanjing Kexue Xuebao/Journal of Agro-Environment Science. 2020;39:1916-24. https://doi.org/10.11654/jaes.2020-0390
- Saenen E, Horemans N, Vanhoudt N, Vandenhove H, Biermans G, Van Hees M, Wannijn J, Vangronsveld J, Cuypers A. Oxidative stress responses induced by uranium exposure at low pH in leaves of Arabidopsis thaliana plants. Journal of Environmental Radioactivity. 2015 Dec 1;150:36-43. https://doi.org/10.1016/j.jenvrad.2015.07.021
- .Bleise, A.; Danesi, P.R.; Burkart,W. Properties, use and health effects of depleted uranium (DU): A general overview. J. Environ. Radioact. 2003, 64, 93–112
- Brugge, D.; de Lemos, J.L.; Oldmixon, B. Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: A review. Rev. Environ. Health 2005, 20, 177–193.
- Zhang, L., Chu, J., Xia, B., Xiong, Z., Zhang, S., & Tang, W. (2022). Health Effects of Particulate Uranium Exposure. Toxics, 10(10), 575.
- United Nations Scientific Committee on the Effects of Atomic Radiation. (2017). Sources, effects and risks of ionizing radiation, united nations scientific committee on the effects of atomic radiation (UNSCEAR) 2016 report: report to the general assembly, with scientific annexes. United Nations.
- Fisenne IM, Perry PM, Decker KM, Keller HW. The daily intake of 234,235,238 U, 228,230,232 Th and 226,228 Ra by New York City residents. Health Physics. 1987 Oct 1;53(4):357-63. https://doi.org/10.1097/00004032-198710000-00002
- Priest ND. Toxicity of depleted uranium. The Lancet. 2001 Jan 27;357(9252):244-6. https://doi.org/10.1016/S0140-6736(00)03605-9
- Cothern CR, Lappenbusch WL. Occurrence of uranium in drinking water in the US. Health physics. 1983 Jul 1;45(1):89-99.
- Hamilton EI. The concentration of uranium in man and his diet. Health physics. 1972 Feb 1;22(2):149-53.
- Sullivan MF, Ruemmler PS, Ryan JL, Buschbom RL. Influence of oxidizing or reducing agents on gastrointestinal absorption of U, Pu, Am, Cm and Pm by rats. Health physics. 1986 Feb 1;50(2):223-32. https://doi.org/10.1097/00004032-198602000-00006
- La Touche YD, Willis DL, Dawydiak OI. Absorption and biokinetics of U in rats following an oral administration of uranyl nitrate solution. Health physics. 1987 Aug 1;53(2):147-62. https://doi.org/10.1097/00004032-198708000-00005
- Harduin JC, Royer P, Piechowski J. Uptake and urinary excretion of uranium after oral administration in man. Radiation protection dosimetry. 1994 May 1;53(1-4):245-8. https://doi.org/10.1093/rpd/53.1-4.245
- ATSDR T. ATSDR (Agency for toxic substances and disease registry). Prepared by clement international corp., under contract. 2000;205:88-0608.
- Fisenne IM, Perry PM, Harley NH. Uranium in humans. Radiation Protection Dosimetry. 1988 24(1-4); 127-131. https://doi.org/10.1093/oxfordjournals.rpd.a080256
- Banerjee S, Kundu A, Dhak P. Bioremediation of uranium from waste effluents using novel biosorbents: a review. Journal of Radioanalytical and Nuclear Chemistry. 2022 Apr 22:1-27. https://doi.org/10.1007/s10967-022-08304-2
- Wise SS, Thompson WD, Aboueissa AM, Mason MD, Wise JP. Particulate depleted uranium is cytotoxic and clastogenic to human lung cells. Chemical research in toxicology. 2007 May 21;20(5):815-20. https://doi.org/10.1021/tx700026r
- Khan F, Pattanayak SK, Verma PR, Dewangan PK. Biofabrication of graphene QDs as a fluorescent nanosensor for detection of toxic and heavy metals in biological and environmental samples. InSmart biosensors in medical care 2020 Jan 1 (pp. 139-152). Academic Press. https://doi.org/10.1016/B978-0-12-820781-9.00008-5
- Tang N, Liang J, Niu C, Wang H, Luo Y, Xing W, Ye S, Liang C, Guo H, Guo J, Zhang Y. Amidoxime-based materials for uranium recovery and removal. Journal of materials chemistry A. 2020;8(16):7588-625.
- Russell JJ, Kathren RL. Uranium deposition and retention in a USTUR whole body case. Health physics. 2004 Mar 1;86(3):273-84.
- Wei Y, Jin L, Li Z, Liu J, Wang L, Pi X, Yin S, Wang C, Ren A. Levels of uranium and thorium in maternal scalp hair and risk of orofacial clefts in offspring. Journal of environmental radioactivity. 2019 Aug 1;204:125-31. https://doi.org/10.1016/j.jenvrad.2019.04.007
- Craft ES, Abu-Qare AW, Flaherty MM, Garofolo MC, Rincavage HL, Abou-Donia MB. Depleted and natural uranium: chemistry and toxicological effects. Journal of Toxicology and Environmental Health, Part B. 2004 Jul 1;7(4):297-317. https://doi.org/10.1080/10937400490452714
- Brugge D, Buchner V. Health effects of uranium: new research findings. https://doi.org/10.1515/REVEH.2011.032
- Katz SA. The chemistry and toxicology of depleted uranium. Toxics. 2014 Mar 17;2(1):50-78. https://doi.org/10.3390/toxics2010050
- Ansoborlo E, Prat O, Moisy P, Den Auwer C, Guilbaud P, Carriere M, et al. Actinide speciation in relation to biological processes. Biochimie. 2006 Nov 1;88(11):1605-18.
- Anke M, Seeber O, Müller R, Schäfer U, Zerull J. Uranium transfer in the food chain from soil to plants, animals and man. Geochemistry. 2009 Feb 18;69:75-90. https://doi.org/10.1016/j.chemer.2007.12.001

This work is licensed under a Creative Commons Attribution 4.0 International License.









