Lupane-Type Triterpenoids and Sterol from Zizyphus Spina Christi Grown in Sudan
Nawal Mohamed Ibrahim Elnagar* 1,2 and Barakat Mohammed Modawi3
1Chemistry Department, Faculty of Education, University of Kordofan.Sudan.
2Chemistry Department, Faculty of Science, University of Hafr Albatin. KSA.
3Chemistry Department, Faculty of Science, University of Khartoum.Sudan.
Corresponding author Email : nawalelnagar@yahoo.co.uk
DOI : http://dx.doi.org/10.13005/ojc/320215
Article Received on :
Article Accepted on :
Article Published : 23 Apr 2016
The fine powder of the shade-dried root bark of Zizyphus spina Christi [Rhamnaceae] was exhaustively extracted with aqueous ethanol at room temperature. Fractionation, isolation and purification were carried out with column chromatography. Characterization and identification of the isolated pure components were based on their spectroscopic data and comparison with data cited in literature. As a result four new compounds, three of them are lupane-type tritepenoids and the fourth compound is sterol were identified. These compounds are lupeol, betulinaldehyde, betulin and β-sitosterol.
KEYWORDS:Zizyphus spina Christi; Root bark; Lupeol; Betulinaldehyde; β- Sitosterol Betulin
Download this article as:| Copy the following to cite this article: Elnagar N. M. I, Modawi B. M. Lupane-Type Triterpenoids and Sterol from Zizyphus Spina Christi Grown in Sudan. Orient J Chem 2016;32(2) |
| Copy the following to cite this URL: Elnagar N. M. I, Modawi B. M. Lupane-Type Triterpenoids and Sterol from Zizyphus Spina Christi Grown in Sudan. Orient J Chem 2016;32(2). Available from: http://www.orientjchem.org/?p=15446 |
Introduction
Throughout human history, natural products have been widely used as remedies1-5 to cure and treat various illnesses. Humans are continuously learning more about the indispensible therapeutic properties of natural products as well as becoming conscious of the importance of a healthy life style-to gain life quality.
An impressive amount of natural substances has been high-lighted by the media3,6 due to their wide-ranging properties. Among these substances are the following lupane- type pentacyclic triterpenoids: Lupeol (1), a significant lupane- type pentacyclic triterpenoid is a common constituent of grape, bazel nut, olive oil, mango pulp and it was isolated from the stem bark of Cralaeva nurvalis6 [capparidacaea]. Lupeol was reported to be a constituent of the white part of birch bark [Betula papyfera] and red alder [Alnus rubra]. 7-9
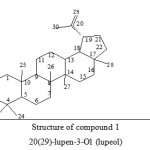 |
Figure 1: Structure of compound 1 20(29)-lupen-3-Ol (lupeol) Click here to View figure |
Lupeol exhibits a broad spectrum of biological activities 7 and can be used as a chemo- preventive substance to avoid several diseases.
Betulinaldehyde (2) was isolated from many plant species10 and it was the most intensively studied constituent of the birch bark triterpenoids because of its unique anti- cancer and anti HIV bioactivity.11,12
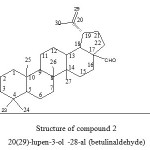 |
Figure 2: Structure of compound 2 20(29)-lupen-3-ol -28-al (betulinaldehyde) Click here to View figure |
Betulinic acid (3) together with lupeol, betulinaldehyde and betulin were isolated from the stem bark of Tectona grandis10, Betulinic acid was also isolated from the root bark of Z. spina Christi.13-16
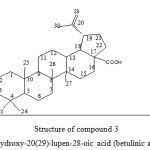 |
Figure 3: Structure of compound 3 3-hydroxy-20(29)-lupen-28-oic acid (betulinic acid) Click here to View figure |
Betulinic acid was synthesized from betulin by selective oxidation.17The reaction required the choice of the proper oxidizing reagent and conditions so that the oxidation reaction takes place predominantly at the primary C-28 hydroxyl group leaving the secondary hydroxyl group at C-3 in fact. Betulinic acid can then be easily obtained from the resulting betulinaldehyde.
Betulin(4), a pentacyclic triterpene was obtained for the first time in 1788 from birch bark [Betula papyfera]8 Betulin occurs in large amounts in the free form in the outer bark of white birch 9and it was isolated from alnus species, Trochoden dron, Vicia faba and many other plants.18
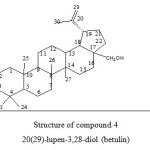 |
Figure 4: Structure of compound 4 20(29)-lupen-3,28-diol (betulin) Click here to View figure |
Since the 1970s, intensive and extensive research has been committed to explore more about the medicinal application of betulin and its derivatives, in particular in their anti-tumor and anti-HIV biological activities.19,20
Betulin is commonly used in medicine, pharmaceutical, perfumes manufacturing and in food and chemical industries.21
β-sitosterol (5) the commonest sterol of higher plants which is widely distributed in plants and also occurs in marine organisms.22-24
β-sitosterol is an important anti-hyper cholesterol, an estrogenic and a hypolipidemic agent. It shows antibacterial and antifungal properties.23-25
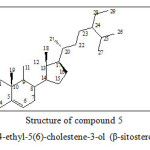 |
Figure 5: Structure of compound 5 24-ethyl-5(6)-cholestene-3-ol (β-sitosterol)
|
Materials and Methods
The plant Material
The plant Z. spina Christi was collected from Kordofan area (Sudan) and identified by the Botany department, Faculty of science University of Khartoum. The root bark was carefully peeled, shade-dried and finally powdered.
Chemicals
The following either analytical grade or carefully purified and perfectly dried chemicals were used. Petroleum ether (pet.ether)[60-80ºC], ethanol, methanol, acetone, dichloromethane (DCM), silica gel for column chromatography [100-200 mesh], silica gel for thin-layer chromatography (TLC) GF254 and anhydrous sodium sulphate.
Instruments
The following spectroscopic techniques were used wherever needed:
- Ultra Violet and Visible absorption Spectroscopy [UV\Vis].
- Infrared Spectroscopy [IR].
- Mass Spectroscopy[ Electron- Impact Mass Spectrometry EI-MS].
- Nuclear Magnetic Resonance Spectroscopy [NMR].
(a) One Dimensional NMR Spectroscopy, Proton Nuclear Magnetic Resonance[1HNMR], carbon-13 Nuclear Magnetic Resonance [13CNMR] and Destortionless Enhancement through Polarization Transfer [DEPT].
(b) Two Dimensional NMR Spectroscopy, Proton Correlated Spectroscopy [COSY], Nuclear Over-houser Effect Spectroscopy [NOESY], Hetronuclear Single Quantum Coherence Spectroscopy [HSQC] and Hetronuclear Multiple bond Correlation Spectroscopy [HMBC].
Methods
Extraction
The fine-dried powder of the root bark of Z. spina christiwas exhaustly extracted with 80% ethanol for 72 hours at room temperature with frequent shaking. The aqueous ethanol extract was filtered through Watman No. 42 filter paper. The solvent was carefully removed, as completely as possible; under reduce pressure using a rotatory evaporator.
The wet crude residue was then dissolved in ethanol and perfectly dried with anhydrous sodium sulphate. The ethanol solution of the crude extract was filtered as before and then centrifuged. The solvent was carefully removed and a mean percentage yield of 28 was obtained.
Column Chromatography
A glass column [120x8cm] well packed with silica gel [100- 200 mesh] in petroleum ether was used for the separation of the components of the crude extract. The crude residue was completely dissolved in ethanol and thoroughly mixed with silica gel. Ethanol was completely removed and the silica gel containing the crude extract was carefully packed on the top of the column.
The column was patiently eluted first with pet.ether followed by successive elution with [5:1], [2:3] and [1:3] pet.ether\DCM solvent mixtures. All fractions obtained from each of the above three successive elutions were carefully monitored with thin-layer chromatography and similar fractions from each successive elution were combined. Three, A, B and D combined fractions were obtained from the above successive elutions. Each of the combined fractions was perfectly dried and filtered. Careful removal of the solvent mixtures from each resulted in the formation of a yellow oily substance from A, a white grey solid from B and a pale green sticky material from D.
Isolation and purification of components
A glass column [40 x1 cm], packed with the same silica gel in pet.ether was used for the separation of the components of the yellow oily substance. The crude substance was dissolved in ethanol and thoroughly mixed with silica gel. After complete removal of ethanol the silica gel containing fraction A was carefully packed on the top of the column.
The column was eluted first with pet. ether\acetone [9:1] and finally with pet. ether\acetone[ 1:1]. Thin-layer chromatography monitoring of all fractions obtained led to the accumulation of two combined fractions [A1 and A2].
Removal of solvent mixture from each gave a white solid in both cases. Both white solids [A1 and A2] were crystallized from pet.ether\acetone [1:1]. A1 is white needles, m.p 216-220ºC whereas A2 is white crystals, m.p 188-190ºC.
The white grey solid was similarly chromatographedusing a glass column [80 X 2cm], packed with the same stationary phase in pet.ether. The column was carefully eluted first with pet.ether\acetone [9:1] and finally with pet.ether\acetone [3:7] solvent mixtures. Thin-layer monitoring of all fractions obtained resulted in the accumulation of similar fractions in two combined fractions, B1 and B2. The two combined fractions [B1 and B2] were perfectly dried separately, filtered and centrifuged.
When the solvent mixture was completely removed from each combined fraction, the resulting solids B1 and B2 were both separately crystallized from DCM and pet.ether\acetone[2:3], B1 gave white needles, m.p. 282-284 ºC whereas B2 gave a white solid with pleasant smell, m.p. 140-143ºC.
A glass column [60X2cm] packed with the same silica gel in pet.ether was used for the separation of the components of the pale green sticky material [D]. The column was successively eluted with pet.ether\acetone[9:2] and [3:8]and finally with acetone\methanol [1:1] and [1:9]. TLC monitoring of all fractions led to the accumulation of only one main combined fraction. Perfect drying of this combined fraction, filtration, centrifuge and complete removal of the solvent mixture, gave a white solid D2 which was crystallized from acetone\ methanol [2:3], m.p. 250-2520C.
Spectroscopic Studies
The isolated and purified components [ A1, A2, B1, B2 and D2 ] were separately subjected to the following spectroscopic investigations using the relevant technique accompanied by the proper interpretation.
Component A1
UV λmax (CHCl3) 242nm.EI-MS, M/Z. 426.3, M+: 411.4, 315.3, 297.2, 218.2, 207.2, 189.2, 175.1, 147.1, 135.1, 121.1, 95.1, 81.1, 69.1, 55.0, 43.0.IR vmax. (KBr) (cm-1), 3346 (OH), 2925 (C-H), 1452 (C=C), 1381 (CH3-C), 1038 (C-O), 880 (C-H).
1H NMR (300MHZ, CDCl3), ppm); shows the protons chemical shifts at 4.66 (1H,d), at 4.54 (1H,d), at 3.17 (1H,dd), at 2.36 (1H,td) and seven singlet methyl protons at 0.74, 0.76, 0.81, 0.92, 0.94, 1.02 and 1.67. 1H – 13C HSQC (400MHZ, CDCl3) δ(ppm); shows that the two protons at 4.66 and 4.54 are bonded to the same carbon atom at δC 109.0) ppm and the proton at δH 3.17 is bonded to a carbon atom at δC 79.0) δ ppm.1H-1H COSY (400MHZ, CDCl3) δ(ppm); shows that H29 is correlated to H30. H29(a&b)are correlated to each other. H2 is correlated to H1and H3, and H21 is correlated toH19 and H22.1H – 13C HMBC (400MHZ, CDCl3) δ(ppm); shows that H30 is correlated to C29, C20& C19. H27 is correlated to C13, C14 & C15.H26 is correlated to C9 &C14. H25 is correlated to C1,C8 , C9, C10&C11. H24 is correlated to C3, C4, C5 &C10. H23 is correlated to C3, C4 &C5and H18 is correlated to C13, C17, C19, & C20.1H-1H NOESY (400MHZ, CDCl3) ppm); shows correlation between H3 and Me(23&24).
Component A2
UVλmax (CHCl3): 241.0nm. El-MS M/Z. 440.1, M+:426.1, 411.1, 379.1, 315.1, 207.1, 189.1, 175.1, 161.1, 135.1, 121.0, 95.1, 81.1 and 55.0. IR vmax. (KBr) (cm-1): 3411 (OH), 2939 (C-H), 1724 (C=O), 1641 (C=C), 1453-1379 (CH3-C), 1039 (C-O)and 885 (C-H).
1HNMR (300 MHZ,CDCl3) δ(ppm); shows the protons chemical shifts at δH4.73 (1H,s), at 4.61 (1H,s), at δH3.16 (1H,dd), at δH2.98 (1H,m) and six singlet methyl protons at 0.73, 0.86, 0.83, 0.94, 0.95 and 1.67. 1H – 13C HSQC (400MHZ, CDCl3) δ(ppm); shows that the two protons at δH 4.73 and δH 4.61 are bonded to δC110.0) carbon, the proton at δH 3.16 is bonded to δC77.2) carbon. 1H-1H COSY (400MHZ, CDCl3) δ(ppm); shows that H29 is correlated to H30. H29(a&b)are correlated to each other. H9 is correlated to H11and H21 is correlated toH19 &H22.1H–13C HMBC (400MHZ, CDCl3) δ (ppm); shows that H30 is correlated to C29, C20& C18. H27 is correlated to C13, C14 & C15. H26is correlated to C9 &C14. H25is correlated to C1, C8 , C9&C10. H24is correlated to C3, C4, C5 &C10. H23is correlated to C3, C4 &C5. H18is correlated to C17, C20 & C29.1H-1H NOESY (400MHZ, CDCl3) ppm); shows the correlation between H3 and Me23&24.
Component B1
UVλmax CHCl3) 209.0 nm.E1-MS M/Z. 456.2, M+: 438.2, 423.2, 410.3, 395.2, 316.3, 302.3, 259.3, 248.3, 207.3, 189.3, 175.4, 161.3, 135.4, 95.4, 81.4, 69.4, 55.4, 43.4.
IR vmax.(KBr) (cm-1): 3450 (OH), 2937 (C-H), 1686 (C=O), 1449 (C=C), 1378 (CH3-C),1189 (C-O), 883 (C-H).1H NMR (400 MHZ, CDCl3) δ (ppm); shows the chemical shifts of protons at δH 4.71 (1H,s), at δH 4.58 (1H,s), at δH3.17 (1H,dd), at δH2.98 (1H,m) and six singlet methyl protons at δH 0.73, 0.80, 0.92, 0.94,0.96 and 1.68.
1H – 13C HSQC of component B1 (400 MHZ, CDCl3) δ(ppm); shows that the two protons at δH 4.71 and δH 4.58 are bonded to δH 110.0) carbon, the protonat δH 2.98 is bonded to δC 47.0) carbon and the proton at δH3.17 (1H,s) is bonded to δC79.0) carbon.1H-1H COSY (400 MHZ, CDCl3) δ(ppm); shows that H29 is correlated to H30. H29(a&b)are correlated to each other. H3 is correlated to H2. H21 is correlated toH19 &H22 and H15 is correlated toH16.1H – 13C HMBC (400 MHZ, CDCl3) (ppm); shows that H30 is correlated to C29, C20& C19. H27 is correlated to C13, C14 & C15. H26 is correlated to C9 &C14. H25 is correlated to C1, C8 , C9&C10. H24 is correlated to C3, C4, C5, C10, &C23. H23 is correlated to C3, C4 C5, C6, & C24 and H18 is correlated to C13, C17, C20, C28 & C29.
1H-1H NOESY (400 MHZ, CDCl3) δ(ppm); shows the correlation between H16 and (δH2.2) H22 (δH1.9).
Component B2
EI-MS M/Z 414.3: M+396.3, 381.3, 351.2, 329.2, 303.2, 289.2, 273.2, 255.2, 199.1, 173.1, 159.1, 145.0, 119.0, 107.0, 81.0, 69.0, 55.0.IR vmax (KBr) cm-1 3363 (OH), 2925 (C-H), 1687.6 (C=O) , 1458.0 (C=C), 1377 (CH3– C), 1230.5 (C-O), 1037.6 (C-O) and 883.3 (C-H).
1H NMR (300 MHZ, CDCl3 + CD3OD) δ(ppm); shows the protons chemical shifts at δH 5.23 (1H,s), at δH 3.4(1H,d), at δH 3.16 (1H,d), and six singlet methyl protons at δH 0.57, 0.68, 0.70, 0.72, 0.80 and 0.91.13C NMR (500 MHZ, CDCl3 + CD3OD) δ(ppm), shows the 13C chemical shifts of C3 δCat (70.5 ppm), of C5 δC (140.8ppm) and of C6 at (121.7 ppm).1H – 13C HSQC (500 MHZ, CDCl3 + CD3OD) δ(ppm), shows that the proton at δH 5.23 is bonded to δC 121.8 carbon and the two protons at δH 3.4 and at δH 3.16 are bonded to 70.5 carbon.1H-1H COSY (500 MHZ, CDCl3 + CD3OD) ppm); shows that H6 is correlated to H7. H3(a&b)are correlated to each other, H3 is correlated to H2&H4. H20 is correlated to H21 &H22, and H24 is correlated to H25.1H – 13C HMBC (500 MHZ, CDCl3 + CD3OD) ppm); shows that H28 is correlated to C23, C24,C25& C29. H21 is correlated to C17, C20 & C22. H20 is correlated to C23 &C24. H19 is correlated to C1, C5 , C9&C10. H18 is correlated to C11, C12, C13, C14,&C17. H8 is correlated to C9. H7 is correlated toC9, C11,C13,&C14. H6 is correlated toC4, C7, C8,&C10, andH4 is correlated to C1, C2, C3,C5 &C6.1H-1H NOESY (500 MHZ, CDCl3 + CD3OD) δ(ppm); shows the correlation between H6(δH 5.23), H4 (δH2.25) and H7 (δH1.87),between H4-a (δH2.25) and H4-b (δH 2.11) between H7 (δH 1.87) and H8 (δH 1.35) and between H3 (δH 3.4) H4 (δH 2.25) and H19 (δH0.91).
Component D2
UV λmax (CHCL3) 442.0 nm. EI-MS M/Z. 442.3, M+: 424.3, 411.3, 302.2, 288.2, 248.1, 207.7, 189.1, 175.1, 147.1, 135.1, 95.0, 81.0, 55.0. IR vmax (KBr) (cm-1): 3417.8 (OH), 2927.4 (C-H), 1447.9 (C=C), 1378.3 (CH3-C), 1032.2 (C-O) and 882.9 (C-H).1HNMR (300 MHZ, (CHCL3) δ(ppm); shows protons chemical shifts at δH 4.66 (1H,s), at δH 4.71 (1H,s), at δH 3.71 (1H,dd), three alcoholic protons at δH 4.58 (1H,t) δH 3.78 (1H,d), δH 4.71 (1Hs), at δH 3.71 (1H,dd), three alcoholic protons at δH 4.58 (1H,t) δH 3.78 (1H,d), δH 3.3(1H,d) one proton at δH 2.36 (1H,dt) and six singlet methyl protons at δH 0.74, 0.80, 0.92, 0.94, 1.0 and 1.68.
1H – 13C HSQC (400 MHZ, CDCl3)δ (ppm); shows that the two protons at δH 4.66 & 4.71 are bonded to (δC 110.0) ppm carbon, a proton at δH 3.17 is bonded to δC 79.0 ppm carbon and two protons at δH 3.3 & δH3.78 are bonded (δC 60.5 ppm) carbon.
1H-1H COSY (400 MHZ, CDCl3) (ppm); shows that H29 is correlated to H30. H29(a&b)are correlated to each other. H3 is correlated to H2. H21 is correlated toH19 &H22 and H15 is correlated toH16.1H – 13C HMBC (400 MHZ, CDCl3) δ (ppm); shows that H30 is correlated to C29, C20& C19. H27 is correlated to C13, C14 & C15. H26 is correlated to C9 &C14. H25 is correlated to C1, C5, C9&C10. H24 is correlated to C3, C4, C5 &C23. H23 is correlated to C3, C4, C5 &C24, and H19is correlated to C18, C20, C21, C29 & C30.
1H-1H NOESY (400 MHZ, CDCl3) δ (ppm); shows a correlation between H3 (δH 3.17) and Me23&24 (δH 0.94 & 0.74 ) and between H18 (δH 1.6) and H28(a&b) (δH 3.3 & 3.78).
Results and Discussion
Z. spina christi family Rhamnaceae is widely distributed in the tropical and subtropical regions of the World. The tree is a multipurpose plant,widely used in folk medicine 21 and its delicious fruit22 has a limited commercial value.
The fine powder of the shade-dried root bark was exhaustively extracted with aqueous ethanol. The dry crude extract [28% mean yield], was carefully and patiently subjected to intensive and extensive fractionation using column chromatography.
Subsequent monitoring with thin-layer chromatography and refractionation of the resulting combined similar fractions led to the isolation of five components referred to as A1, A2, B1, B2 and D2. Which were vigorously purified.
Characterization and structure elucidation of the isolated and purified above components were based on different spectroscopic data including 1D and 2D NMR spectroscopy.
Careful study of the proton nuclear magnetic resonance spectroscopic data for each of the components A1, A2, B1, B2 and D2 revealed that these components have triterpene skeletons. The HSQC and COSY spectroscopic investigations showed that the above triterpene skeletons are of lupane-type.
Proper interpretation of the infrared analytical spectroscopy and the Hetronuclear Multiple bond Correlation Spectroscopy led to the possible suggestions that the structures of the above components might be: 20(29)-lupen-3-ol, 20(29)-lupen-3-ol-28-al, 20(29)-lupen-3-ol-28-oic acid, 20(29)-lupen-3,28-diol respectively. Their NOESY spectroscopic correlations data confidently confirm the above suggested structures. Identification of these components was based on comparing their spectroscopic data with literature data given for triterpenes isolated from plant materials. These components are lupeol, betulinaldehyde, betulinic acid and betulin respectively.
Study of the 1HNMR, HSQC and COSY of spectroscopic data indicate that component B2 is Sterol. Careful interpretation of the infrared resonance spectra and the HMBC correlation spectra showed that the structure of B2 might be 24-ethyl-5(6)-cholestene-3-ol. The NOESY correlation spectra of B2 confirmed the determined structure and comparison with literature data given for steroids isolated from plant species proved that B2 is β-sitosterol. Thus all the isolated components are known compounds previously obtained from other plants.
To the best of the author’s knowledge, lupeol, betulinaldehyde, betulin and β-sitosterol were isolated for the first time from the root bark of Z. spina Christi grown in Sudan.
Acknowledgement
We are highly indebted to Prof. Mohammed Igbal Choudhary and Dr. Achyut Adhikari and all members of Hussin Ebrahim Jamal (HEJ) research institute of chemistry, International Center for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi-Pakistan, for their indispensible help and constructive advices.
References
- Sofowora, Medicinal plants and Traditional Medicine in Africa, John Wiley and Sons limited. (1982)
- Abdel wahhab, M. A.; Omara, E. A; Abdel Gali, M. M.; Hassan, N. S.; Nada, S. A.; Saeed, A.; Elsayed M. M. African Journal of Traditional, Complementary and Alternative Medicines. 2007, 4, 248-256.
- Sofowora, A. Medicinal Plants and Traditional Medicines in Africa. Chichester John, Willey & Sons New York; 256. (1993).
- Steven, M.; Russell, J. C.; Molyneux, J.; Bioactive Natural Components of the petroleum ether fraction from the root bark ethanol extract using GC\MS l Products Detection, Isolation and Structural Determination Taylor & Francis, USA, (2008).
- New man, D.J; Cragg G.M. Journal of Natural Products. 2007, 70, 461-477.
- Natural Products Reports, New York. 1985, 26, 299- 306.
- International Journal of Biochemical and Pharmaceutical sciences. Global Science Books (2009).
- Basa Vara, J.a. ; H. S. J. Pharm. Science and Res., 2010, 3, 13 – 15.
- Agar Wal, R. B. ; and Rangari, V. D. Indian Journal of Phamacology. 2003, 35,284 -287.
- Indian Journal of Pharmaceutical Sciences. 1988, 50,124 -125,.
- Slinivasan, T. BioOrganic and Medicinal Chemistry Letters. 2002, 12, 2803 -2806.
CrossRef - Shah, A. H; Pandey, V.B ; Eckhard, G. , Tschesche, R. J. Nat. Prod. 1985 48, 555-558.
CrossRef - Jan Sarek; J. Med. Chemistry. 2003, 71, 5402 -5415.
CrossRef - Shah ,A.H. ; Ageel, A.M.; Tarig, M.; Mossa, J.S.; Al-Yahya, M.A. Chemical constituents of the stem bark of Zizyphus spina Christi. Fitoterapia. 1986 ,57,452-454,.
- Anthony, C.; Dweck, F.L.S . Personal Care Magazine . 2005, 2-5.
- Adzu, B.; Haruna, A.K.; Ilyas M. ; Pateh, U.U; Tarfa, F.D.; Chindo, B.A ; Gamaniel, A .S .Journal of Pharmacy and Nutrition Sciences. 2011, 1, 48 -53.
CrossRef - Hongyan Liu; Synthesis of Betulinic acid from Betulin; M.Sc of science; Natural library of Canada; Acquisitions and Bibiographic Servicas; 2001.
- Yunusoy, M. S. Russion Plant, 6, pp. CODEN, RUXX, 67, RU 2270202 C1 20060220.
- EL Znhmer, D. V. XLI 2Q. Drugs. 2007, 7,359 – 373.
- Fujioka T.; Kashiwada Y.; Kilkuskie R. E.; Consentino L. M.; Ballas L. M.; Jiang J. B.; Janzen W. P. Journal of Natural Products. 1994, 57, 243 – 247.
CrossRef - www/Natural News. Com., Natural health, Natural living, Natural News.
- Sudhersan C.; Hussain J. Turk J Bot. 2003, 27,167-171.
- Steele, J. A. JOC. 1963, 28, 571-572.
CrossRef - Mastomato, T. Phytochemistry. 1983, 22,2622-24.
CrossRef - Aizawa, K. Org Mass Spectrom. 1974, 9, 470 -79.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









