Solvent-Free Synthesis of New Bis(Benzimidazole-2-Alkylthio) Polyethylene Oxides Starting from Dithiols
A. Akremi1,2 and A. Noubigh2
1,2Department of Chemistry, Faculty of Sciences, Northern Border University, Arar 91431, Kingdom of Saudi Arabia.
1Department of Chemistry, Faculty of science, El Manar University, Tunis, Tunisia.
2Laboratory of Physical Chemistry of Materials , Preparatory Institute for Scientific and Technical Studies of La Marsa, 2070, Carthage University, Tunisia.
Corresponding Author E-mail: akrimimi@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330209
Article Received on : November 24, 2016
Article Accepted on : January 22, 2017
Polyethylene oxide (PEO) could be incorporated in molecular structure by several ways. A series of diacid S-alkylpolyethylene oxides 4a-i have been prepared from dithiols 1a-c through multistep reaction sequence. Novel symmetric bis-benzimidazoles (polyethylene oxide) 6a-i have been synthesized over green route. The condensation of the title compounds 4a-i on o-phenylenediamine 5, via the further deamination reaction and without catalyst, afforded the corresponding bis-benzimidazoles 6a-i in acceptable yields. The new compounds were established on the basis of their elemental analysis, IR, 1H NMR, 13C NMR and mass spectral data.
KEYWORDS:Green route; bis-benzimidazoles polyethylene oxides; condensation; NMR
Download this article as:| Copy the following to cite this article: Akremi A, Noubigh A. Solvent-Free Synthesis of New Bis(Benzimidazole-2-Alkylthio) Polyethylene Oxides Starting from Dithiols. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Akremi A, Noubigh A. Solvent-Free Synthesis of New Bis(Benzimidazole-2-Alkylthio) Polyethylene Oxides Starting from Dithiols. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31017 |
Introduction
Poly(ethyleneoxide) PEO is a biocompatible eroding polymer available in a various molecules. PEO have recently received wide attention as sustained-release bioadhesive platform due to its safety, ease of processing and possibility to control drug release. In medicine, the polymer has been incorporated in several drugs such as oral sustained-release tablets[1-5] and ocular inserts.[6] in addition, it has been shown that particles with poly(ethylene oxide) surfaces have great potential as long circulating systems after intravenous administration.[7-11]
Homobifunctional polyethylene oxides derivatives are synthetic polyethers containing two of the same type of functional group, available in a range of molecular weights and various functionalities, such as amines, maleimides, azides, thiols, and NHS esters. Hence, they can be used for many applications including surface functionalization of a various nanoparticles[12,13] and as cross-linkers for proteins, peptides, and other biomolecules.[14,15].
Furthermore, compounds containing benzimidazole core could exhibit a wide range of various biological activities including anti-cancer,[16] bactericidal,[17] fungicidal,[18,19] analgesic[20] and anti-viral properties.[21] Thus, recognition of the great interest of bis-benzimidazoles has led to a considerable effort being invested in designing and developing reliable inexpensive and green synthetic routes for these compounds.[22-26]
In previous work,[27, 28] we reported the synthesis of well-known biologically important heterocycles starting from thiols. In the same context, we have developed an efficient one-pot solvent-free synthesis of a series of symmetric bis(benzimidazole-2-alkylthio) polyethylene oxide derivatives 6a-i in acceptable yields.
Results and Discussion
The intermediates diesters 3a-i were prepared in basic medium from reaction of dithiols 1a-c with various methyl esters bromide 2a-c.[28] The saponification of compounds 3a-i was performed in boiling alkaline aqueous solution, giving after acidification the corresponding diacids 4a-i (Scheme 1). Product 4a-c were isolated in good yields but 4g-i could not be obtained probably because of the length of polyethylene chain which more soluble in water. All results are given in Table 1.
Structures of compounds 4 were confirmed by NMR and IR spectroscopy. Diacid 4b showed a broad signal at 10.8 ppm which
corresponds to the acidic proton, its 13C NMR spectrum revealed a peak at 179 ppm assigned to carbonyl group. The IR spectra of product 4h displayed a stretching vibration band at 1732 cm-1 due to C=O group.
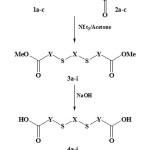 |
Scheme 1: Synthesis of diacid derivatives
|
Table 1: Yields of synthesized diacids 4a-i with various X and Y groups
|
Entry |
X |
Y |
Yield (%) |
|
4a |
-CH2-CH2-O-CH2-CH2– |
-CH2– |
82 |
|
4b |
-CH2-CH2-O-CH2-CH2– |
-CH(CH3)- |
79 |
|
4c |
-CH2-CH2-O-CH2-CH2– |
-CH2-CH2– |
72 |
|
4d |
-CH2-CH2-(O-CH2-CH2)2– |
-CH2– |
58 |
|
4e |
-CH2-CH2-(O-CH2-CH2)2– |
-CH(CH3)- |
62 |
|
4f |
-CH2-CH2-(O-CH2-CH2)2– |
-CH2-CH2– |
57 |
|
4g |
-CH2-CH2-(O-CH2-CH2)3– |
-CH2– |
44 |
|
4h |
-CH2-CH2-(O-CH2-CH2)3– |
-CH(CH3)- |
51 |
|
4i |
-CH2-CH2-(O-CH2-CH2)3– |
-CH2-CH2– |
49 |
Bis-benzimidazole derivatives 6a-i were prepared in acceptable yields without catalyst by reaction of o-phenylenediamaine with various diacids via melting method (Scheme 2). The consumption of diacids was monitored by TLC (5:1, v/v, dichloromethane: petroleum ether) as eluent. This methodology was applied to synthetize various bis(benzimidazole-2-alkylthio) polyethylene oxide derivatives and the results are summarized in Table 2.
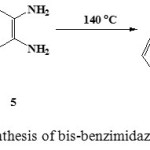 |
Scheme 2: Synthesis of bis-benzimidazole derivatives
|
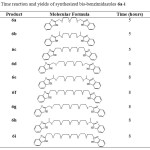 |
Table 2: Time reaction and yields of synthesized bis-benzimidazoles 6a-i Click here to View table |
The effect of the length of polyethylene oxide chain of different substituted diacids has been investigated and the results show that diacids bearing long polyethylene oxide chain reacted slowly with lower yields. At the same time, diacids containing short ethylene oxide chain, such as 6a-c, afford acceptable yields with reduced reaction times. Products were characterized by comparing with authentic sample (IR, NMR and MS). NH signal clearly appeared in the region between δ 12.1 to 13.1 in 1H NMR and quaternary carbon appeared around δ 150-154 in 13C NMR spectra, as expected. In addition, the polyethylene oxide proton signal appears as multiplet at ~3.8 ppm. The molecular ion peak appeared at respective m/z values in GC-MS spectrometry.
These new compounds may be useful intermediates for the synthesis of organic-metal complexes[29-32] or for the preparation of lipophilic crown or azathiacrown ethers bearing bis-benzimidazoles[33, 34] (Scheme 3).
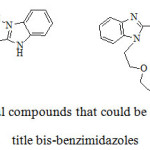 |
Scheme 3: Useful compounds that could be derived from the title bis-benzimidazoles
|
Conclusion
New bis-(benzimidazole-2-alkylthio)ethylene and polyethylene oxides were prepared from various dithiols. The synthesized products may be useful intermediates for the preparation of several interesting well-known compounds such as bis-benzimidazole metal complexes or azathiacrown ethers bearing bis-benzimidazole. The study related to the application of these new bisbenzimidazoles in biological and industrial fields is in progress.
Acknowledgement
The authors wish to acknowledge the approval and the support of this research study by the grant N°. 5-15-1436-5 from the Deanship of the Scientific Research in Northern Border University, Arar, KSA.
Experimental
Apparatus and materials
Progress of the reaction was monitored by thin layer chromatography (0.25 mm thick precoated silica plates: Merck Fertigplatten Kieselgel 60F254), although purification was effected by silica gel column chromatography (Merck Kieselgel 60; 230-400 mesh). Infrared spectra were recorded on a Perkin-Elmer Fouriertransform (FT) Paragon 1000 PC (PerkinElmer, France). 1H-NMR and 13C-NMR spectra were obtained using a Bruker Avance 300 spectrometer (Bruker, France) at 300 and 75MHZ, respectively. Chemical shifts were reported in ppm from internal TMS for 1Hand 13C.The mass spectra were performed on a Shimadzu GC MS-QP 1000EX apparatus (Kyoto, Japan). Elemental analysis was recorded on an Exeter Analytical CE-440 Elemental instrument (Exeter Analytical, UK). Dithiols was purchased from Sigma-Aldrich and used as received. The synthesis of diesters has been described elsewhere.
General method for bis-(benzimidazole-alkylthio)ethylene oxide formation
A mixture of diacid (1 mmol) and ortho-phenylenediamine (1.25 mmol) were added to a dry round-bottomed flask (50 mL). Then, the flask fitted with a condenser was placed in an oil bath and heated at 140°C for 5 h. The reaction was monitored by TLC till the disappearance of the starting diacid. Once the reaction was complete, the mixture was dissolved in alcohol. The pH value of the resulted solution was adjusted to 9-10 with KOH solution to give the crude product, which was purified by flash chromatography on silica gel (5:1, v/v, dichloromethane: petroleum ether) as eluent to afford the samples 6a-i for analysis.
2-((2-(2-((1H-Benzo[D]Imidazol-2-Yl)Methylthio)Ethoxy)Ethylthio)Methyl)-1H-Benzo[D]Imidazole 6a
Light yellow solid; m.p.115°C; 1H-NMR (DMSO-d6/TMS) δ 2.75 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 3.44 (s, 4H, 2 C2-CH2 and C2’-CH2), 3.59 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 12.34 (br, 2H, N1-H and N1′-H); 13C-NMR (δ (ppm), DMSO-d6/TMS): 26.33 (s, C2-CH2 and C2’-CH2), 34.8 (s, 2 O-CH2-CH2-S), 70.9 (s, 2 O-CH2-CH2-S), 115.1 (C4, C7, C4′ and C7′), 121.8 (C5 , C6 , C5′ and C6′), 140.2 (C3a , C7a , C3a’ and C7a’ ) and 153.7; IR (KBr): 3380, 2870, 1590, 1461, 1303, and 1205cm-1;MS [m/z, (%)]: 399.13 [M+H]+ (90); Elemental Analysis: Found, %: C 60.18; H 5.51; N 13.97; C20H22N4OS2, Calculated, %: C 60.34; H 5.57; N 14.07.
2-(1-(2-(2-(1-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy)Ethylthio)Ethyl)-1H-Benzo[D]Imidazole 6b
Light yellow solid; m.p.107°C; 1H-NMR (DMSO-d6/TMS) δ 1.42 (d, 6H, 2CH(CH3), 3JHH = 6.0 Hz), 2.73 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 3.48 (m, 2H, 2CH(CH3)), 3.48 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 12.99 (br, 2H, N1-H and N1′-H); 13C-NMR (δ (ppm), DMSO-d6/TMS): δ 18.5 (s, CH(CH3)), 34.84 (s, 2 O-CH2-CH2-S), 37.29 (s, CH(CH3)), 70.8 (s, 2 O-CH2-CH2-S), 71.2 (s, O-CH2-CH2-O), 115.7 (C4, C7, C4′ and C7′), 122.3 (C5 , C6 , C5′ and C6′), 140.6 (C3a , C7a , C3a’ and C7a’ ) and 153.2; IR (KBr): 3385, 2871, 1592, 1464, 1301, and 1197 cm-1;MS [m/z, (%)]: 427.16 [M+H]+ (100); Elemental Analysis: Found, %: C 61.91; H 6.01; N 13.04; C22H26N4OS2, Calculated, %: C 62.00; H 6.15; N 13.14.
2-(2-(2-(2-(2-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy)Ethylthio)Ethyl)-1H-Benzo[D]Imidazole 6c
Light yellow solid; m.p. 111°C; 1H-NMR (DMSO-d6/TMS) δ 2.76 (t, 4H, 2 CH2, 3JHH = 6.0 Hz), 3.26 (m, 4H, CH2-CH2-C2), 2.69 (t, 4H, 2 S-CH2-CH2-O), 3JHH = 6.0 Hz), 3.65 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 7.1-7.2 (m, 4H, C5-H, C6-H, C5′-H and C6′-H), 7.5-7.6 (m, 4H, C4-H, C7-H, C4′-H and C7′-H), 12.19 (br s, 2H, N1-H and N1′-H); 13C-NMR (DMSO-d6/TMS) δ 27.1 (C2-CH2-CH2 and C2’-CH2-CH2), 30.8 (C2-CH2-CH2 and C2’-CH2-CH2), 35.3 (S-CH2-CH2-O)), 70.5 (S-CH2-CH2-O)), 115.2 (C4, C7, C4′ and C7′), 121.9 (C5 , C6 , C5′ and C6′), 139.2 (C3a , C7a , C3a’ and C7a’ ) and 153.1 (C2 and C2′); IR (KBr): 3378, 2862, 1588, 1454, 1303, and 1201 cm-1; MS [m/z, (%)]: 427.17 [M+H]+ (80); Elemental Analysis: Found, %: C 61.89; H 6.03; N 13.03; C20H26N4OS2, Calculated, %: C 62.00; H 6.15; N 13.14.
1,2-Bis(2-((1H-Benzo[D]Imidazol-2-Yl)Methylthio)Ethoxy)Ethane 6d
White solid; m.p. 91°C; 1H-NMR (DMSO-d6/TMS) δ 2.69 (t, 4H, 2 S-CH2-CH2-O,3JHH = 6.0 Hz), 3.50 (s, 4H, C2-CH2, and C2’-CH2), 3.62 (t, 4H, 2 S-CH2-CH2-O, 3JHH = 6.0 Hz), 3.79 (s, 4H, S-CH2-CH2-O), 13.15 (br, 2H, 2 NH); 13C-NMR (δ (ppm), DMSO-d6/TMS): 26.2 (s, C2-CH2, and C2’-CH2), 35.1 (s, 2 O-CH2-CH2-S), 71.2 (s, 2 O-CH2-CH2-S), 71.5 (s, O-CH2-CH2-O), 115.1 (C4, C7, C4′ and C7′), 122.3 (C5 , C6 , C5′ and C6′), 139.6 (C3a , C7a , C3a’ and C7a’) and 151.3 (C2 and C2′); IR (KBr): 3380, 2872, 1580, 1451, 1299, and 1199 cm-1; MS [m/z, (%)]: 443.15 [M+H]+ (60); Elemental Analysis: Found, %: C 59.62; H 5.88; N 12.59; C22H26N4O2S2, Calculated, %: C 59.76; H 5.92; N 12.67.
1,2-Bis(2-(1-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy) Ethane 6e
White solid; m.p. 88°C; 1H-NMR (DMSO-d6/TMS) δ 1.49 (d, 6H, 2CH(CH3), 3JHH = 6.0 Hz), 2.69 (t, 4H, 2 O-CH2-CH2-S,3JHH = 6.0 Hz), 3.44 (m, 2H, 2CH(CH3)), 3.62 (t, 4H, O-CH2-CH2-S, 3JHH = 6.0 Hz), 3.81 (s, 4H, O-CH2-CH2-O), 12.31 (br s, 2H, N1-H and N1′-H); 13C-NMR (δ (ppm), DMSO-d6/TMS): 18.1 (CH(CH3)) 37.2 (s, CH(CH3)), 35.0 (s, 2 O-CH2-CH2-S), 71.4 (s, 2 O-CH2-CH2-S), 71.2 (s, O-CH2-CH2-O), 115.3 (C4, C7, C4′ and C7′), 122.1 (C5 , C6 , C5′ and C6′), 139.5 (C3a , C7a , C3a’ and C7a’ ) and 151.9 (C2 and C2′); IR (KBr): 3379, 2871, 1579, 1452, 1300, and 1200 cm-1; MS [m/z, (%)]: 471.19 [M+H]+ (70); Elemental Analysis: Found, %: C 61.22; H 6.40; N 11.81; C24H30N4O2S2, Calculated, %: C 61.31; H 6.43; N 11.91.
1,2-Bis(2-(2-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy)Ethane 6f
White solid; m.p. 94°C; 1H-NMR (DMSO-d6/TMS) δ 2.75 (t, 4H, 2 O–CH2–CH2–S, 3JHH = 6.0 Hz), 3.24 (m, 4H, CH2-CH2-C2), 3.66 (t, 4H, O–CH2–CH2–S, 3JHH = 6.0 Hz), 3.83 (s, 4H, O-CH2-CH2-O) 7.1-7.2 (m, 4H, C5-H, C6-H, C5′-H and C6′-H), 7.5-7.6 (m, 4H, C4-H, C7-H, C4′-H and C7′-H), 12.19 (br s, 2H, N1-H and N1′-H); 13C-NMR (DMSO-d6/TMS) δ 27.3 (C2-CH2-CH2 and C2’-CH2-CH2), 30.5 (C2-CH2-CH2 and C2’-CH2-CH2), 35.4 (S-CH2-CH2-O)), 70.5 (S-CH2-CH2-O)), 71.6 (s, O-CH2-CH2-O), 115.2 (C4, C7, C4′ and C7′), 121.9 (C5 , C6 , C5′ and C6′), 139.2 (C3a , C7a , C3a’ and C7a’ ) and 151.1 (C2 and C2′); IR (KBr): 3378, 2862, 1588, 1454, 1303, and 1201 cm-1; MS [m/z, (%)]: 471.18 [M+H]+ (60); Elemental Analysis: Found, %: C 61.18; H 6.38; N 11.84; C24H30N4O2S2, Calculated, %: C 61.31; H 6.43; N 11.91.
2-((2-(2-(2-(2-((1H-Benzo[D]Imidazole–2-Yl)Methylthio)Ethoxy)Ethoxy)Ethoxy)Ethylthio) Methyl) -1H-Benzo[D]Imidazole 6g
Semisolid; 1H-NMR (DMSO-d6/TMS) δ 2.70 (t, 4H, 2 O-CH2-CH2-S,3JHH = 6.0 Hz), 3.48 (s, 4H, 2 C2-CH2, and C2’-CH2), 3.60 (t, 4H, O-CH2-CH2-S, 3JHH = 6.0 Hz), 3.80 (s, 4H, 2 O-CH2-CH2-O), 13.02 (br, 2H, 2 NH); 13C-NMR (δ (ppm), DMSO-d6/TMS): 26.9 (s, C2-CH2 and C2’-CH2), 35.3 (s, 2 O-CH2-CH2-S), 71.4 (s, 2 O-CH2-CH2-S), 72.1 (s, O-CH2-CH2-O), 115.1 (C4, C7, C4′ and C7′), 122.3 (C5 , C6 , C5′ and C6′), 139.6 (C3a , C7a , C3a’ and C7a’ ) and 150.3 (C2 and C2′); IR (KBr): 3370, 2871, 1582, 1450, 1299, and 1202 cm-1;MS [m/z, (%)]: 487.18 [M+H]+ (40); Elemental Analysis: Found, %: C 59.22; H 6.17; N 11.47; C24H30N4O3S2, Calculated, %: C 59.29; H 6.22; N 11.52.
2-(1-(2-(2-(2-(2-(1-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy)Ethoxy)Ethoxy)Ethylthio) Ethyl)-1H-Benzo[D]Imidazole 6h
Semisolid; 1H-NMR (DMSO-d6/TMS) δ 1.50 (d, 6H, 2CH(CH3), 3JHH = 6.0 Hz), 2.70 (t, 4H, 2 O-CH2-CH2-S,3JHH = 6.0 Hz), 3.41 (m, 2H, 2CH(CH3)), 3.60 (t, 4H, 2 O-CH2-CH2-S, 3JHH = 6.0 Hz), 3.81 (s, 4H, 2 O-CH2-CH2-O), 12.55 (br s, 2H, N1-H and N1′-H); 13C-NMR (δ (ppm), DMSO-d6/TMS): 18.7 (CH(CH3)) 37.0 (s, CH(CH3)), 35.1 (s, 2 O-CH2-CH2-S), 71.2 (s, 2 O-CH2-CH2-S), 71.5 (s, O-CH2-CH2-O), 115.5 (C4, C7, C4′ and C7′), 122.2 (C5 , C6 , C5′ and C6′), 140.1 (C3a , C7a , C3a’ and C7a’ ) and 150.5 (C2 and C2′); IR (KBr): 3375, 2872, 1580, 1451, 1301, and 1202 cm-1; MS [m/z, (%)]: 515.21 [M+H]+ (40); Elemental Analysis: Found, %: C 60.61; H 6.59; N 10.81; C26H34N4O3S2, Calculated, %: C 60.73; H 6.22; N 11.52.
2-(2-(2-(2-(2-(2-(2-(1H-Benzo[D]Imidazol-2-Yl)Ethylthio)Ethoxy)Ethoxy)Ethoxy)Ethylthio) Ethyl)-1H-Benzo[D]Imidazole 6i
Semisolid; 1H-NMR (DMSO-d6/TMS) δ 2.74 (t, 4H, 2 O-CH2-CH2-S, 3JHH = 6.0 Hz), 3.26 (m, 4H, CH2-CH2-C2), 3.64 (t, 4H, 2 O-CH2-CH2-S, 3JHH = 6.0 Hz), 3.80 (s, 4H, 2 O-CH2-CH2-O), 7.1-7.2 (m, 4H, C5-H, C6-H, C5′-H and C6′-H), 7.5-7.6 (m, 4H, C4-H, C7-H, C4′-H and C7′-H), 12.94 (br s, 2H, N1-H and N1′-H); 13C-NMR (DMSO-d6/TMS) δ 27.0 (C2-CH2-CH2 and C2’-CH2-CH2), 30.1 (C2-CH2-CH2 and C2’-CH2-CH2), 35.9 (S-CH2-CH2-O), 70.8 (S-CH2-CH2-O), 71.9 (s, O-CH2-CH2-O),, 115.5 (C4, C7, C4′ and C7′), 122.1 (C5 , C6 , C5′ and C6′), 139.4 (C3a , C7a , C3a’ and C7a’ ) and 151.0 (C2 and C2′); IR (KBr): 3376, 2868, 1589, 1451, 1301, and 1200 cm-1; MS [m/z, (%)]: 515.20 [M+H]+ (50); Elemental Analysis: Found, %: C 60.65; H 6.59; N 10.83; C26H34N4O3S2, Calculated, %: C 60.37; H 6.66; N 10.89.
References
- Apicella, A.; Cappello B.; Del Nobile, M. A.; La Rotonda, M. I.; Mensitieri, G.; Nicolais, L. Biomaterials 1993, 14, 83-90.
CrossRef - Cappello, B.; Del Nobile, A. M.; La Rotonda, M. I.; Mensitieri, G.; Miro, A.; Nicolais, L. II Farmaco 1994, 49, 809-818.
- Cappello, B.; De Rosa, G.; Giannini, L. Int. J. Pharm. 2006, 319, 63-70.
CrossRef - Kim, C. J. Pharm. Sci. 1995, 84, 303-306.
CrossRef - Kim, C. Drug Dev. Ind. Pharm. 1998, 24, 645-651.
CrossRef - Di Colo, G.; Burgalassi, S.; Chetoni, P. Int. J. Pharm. 2001, 215, 1-2, 101-111.
CrossRef - Gref, R.; Minamitake, Y.; Peracchia, M. T.; Domb, A.; Trubetskoy, V.; Torchilin, V.; Langer, R. Plenum Press: New York, 1997.
- Griffith, L. G. Acta Mater. 2000, 48, 263-277.
CrossRef - Stolnik, S.; Dunn, S. E.; Garnett, M. C.; Davies, M. C.; Coombes, A. G. A.; Taylor, D. C.; Irving, M. P.; Purkiss, S. C.; Tadros, T. F.; Davis, S. S.; Illum L. Pharm. Res. 1994, 11, 1800-1808.
CrossRef - Holmberg, K.; Tiberg, F.; Malmsten, M.; Brink, C. Colloid. Surface. A 1997, 123-124, 297-306.
CrossRef - Riley, T.; Stolnik, S.; Heald, C. R.; Xiong, C. D.; Garnett, M. C.; Illum, L.; Davis, S. S. Langmuir 2001, 17, 3168-3174.
CrossRef - Aoun, V.; Pagniez, F.; Hildgen, P.; Leclair, G.; Le Pape, P. J. Medical Mycol. 2013, 1, 23, 74-75.
CrossRef - Sasatsu, M.; Onishi, H.; Machida, Y. Int. J. Pharm. 2006, 317, 2, 167-174.
CrossRef - Filpula, D.; Zhao, H. Adv. Drug Deliver. Rev. 2008, 60, 1, 29-49.
CrossRef - Yu, J.; Chen, F.; Wang, X.; Dong, N.; Lu, C.; Yang, G.; Chen, Z. Polym. Degrad. Stabil. 2016, 133, 312-320.
CrossRef - Bhambra, A. S.; Edgar, M.; Elsegood, M. R. J.; Horsburgh, L.; Kryštof, V.; Lucas, P. D.; Mojally, M.; Teat, S. J.; Warwick, T. G.; Weaver, G. W.; Zeinali, F. J. Fluorne Chem. 2016, 188, 99-109.
CrossRef - Keri R. S.; Rajappa C. K.; Patil S. A.; Nagaraja B. M. Pharmacol. Rep. 2016, 68, 6, 1254-1265.
CrossRef - Mohamed, G. G.; Ibrahim, N. A.; Attia, H. A. E. Spectrochimica Acta Part A 2009, 72, 3, 610-615.
CrossRef - Suresh, S.; Karthikeyan, S.; Saravanan, P.; Jayamoorthy, K.; Dhanalekshmi, K.I. Karbala Int. J. Mod. Sci. 2016, 2, 2, 129-137.
CrossRef - Gaba, M.; Gaba, P.; Uppal, D.; Dhingra, N.; Bahia, M. S.; Silakari, O.; Mohan, C. Acta Pharma. 2015, 5, 4, 337-342.
- Pan, T.; He, X.; Chen, B.; Chen, H.; Geng, G.; Luo, H.; Zhang, H.; Bai, C.; Eur. J.Med. Chem. 2015, 95, 500-513.
CrossRef - Mukhopadhyay, C.; Tapaswi, P. K. Tetrahedron Lett. 2008, 49, 6237-6240.
CrossRef - Wang, Y.; Sarris, K.; Sauer, D. R.; Djuric, S. W. Tetrahedron Lett. 2006, 47, 4823-4826.
CrossRef - Mamada, M.; Pérez-Bolívar, C.; Pavel, Anzenbacher, Jr. Org. Lett. 2011, 13, 4882-4885.
CrossRef - Maiti, B.; Chanda, K.; Selvaraju, M.; Tseng, C-C; Sun, C-M. ACS. Comb. Sci. 2013, 15, 291-297.
CrossRef - Suvendu, S.; Sudipto, D.; Papu, B. J. Org. Chem. 2013, 78, 11184-11193.
CrossRef - Akremi, A.; Beji, M.; Baklouti, A. Synth Commun. 2011, 41, 1990-1998.
CrossRef - Akremi, A.; Beji, M.; Baklouti, A. J. Heterocyclic Chem. 2015, 52, 586-591.
CrossRef - Siddiqi, Z. A.; Shahid, A. M.; Khalid, M.; Sharma, P. K.; Siddique, A. J. Mol. Struct. 2013, 1037, 402-411.
CrossRef - Wu, H.; Kou, F.; Jia, F.; Liu, B.; Yuan, J. Acta Cryst. 2011, E67, m1461.
- Tavman, A.; Çinarli, A. Inorg. Chim. Acta 2014, 421, 481-488.
CrossRef - Kopel, P.; Wawrzak, D.; Langer, V.; Cihalova, K.; Chudobova, D.; Vesely, R.; Adam, V.; Kizek R. Molecules 2015, 20, 10360-10376.
CrossRef - Liu, Q-X; Zhao, X-J; Wu, X-M; Guo, J-H; Wang, X-G. J. Organomet. Chem. 2007, 92, 5671–5679.
CrossRef - Álvaro, M.; García, H.; Palomares, E.; Achour, R.; Moussaif, A.; Zniber, R. Chem. Phys. Lett. 2001, 350, 240-246.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









