Antibacterial Activity of Coumarine Derivatives Synthesized from 4-Chloro-chromen-2-one. The Comparison with Standard Drug.
Aziz Behrami1
Faculty of Food Technology, University of Mitrovica, Kosovo
DOI : http://dx.doi.org/10.13005/ojc/300433
Article Received on :
Article Accepted on :
Article Published : 13 Nov 2014
This work reports the synthesis of some new derivatives from 4-Chloro-chromen-2-one and describe the results of antibacterial activity of purified compounds. Compounds 4-Butylamino-chromen-2-one (1a) , 4-Butylamino-2-oxo-2H-chromene-3-sulfonyl chloride (2a) , 4-Butylamino-2-oxo-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (3a), 4-Butylamino-5-ethyl-2-oxo-7-(N'-phenyl-hydrazino)-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (4a) , have been synthesized and characterized using melting points , IR spectra , 1H-NMR and 13C-NMR spectra. The antibacterial activity of synthesized compounds and streptomycin at concentractions of 1mg/ml, 3mg/ml and 5mg/ml , have been evaluated against three strains of bacterial culture; Staphylococcus aureus, E.coli and Klebsiella. The compounds show bacteriostatic and bactericidal activity.
KEYWORDS:4-Chloro-chromen-2-one; coumarine derivatives; antibacterial activity; Staphylococcus aureus; E.coli; Klebsiella; streptomycin
Download this article as:| Copy the following to cite this article: Behrami A. Synthesis, Antibacterial Activity of Coumarine Derivatives Synthesized from 4-Chloro-chromen-2-one. The Comparison with Standard Drug |
| Copy the following to cite this URL: Behrami A. Synthesis, Antibacterial Activity of Coumarine Derivatives Synthesized from 4-Chloro-chromen-2-one. The Comparison with Standard Drug. Orient J Chem 2014;30(4). Available from: http://www.orientjchem.org/?p=5323 |
INTRODUCTION
Starting from 4-Chloro-chromen-2-one (a); derivatives (1a,2a,3a,4a) are synthesized.
Coumarin derivatives are large group of heterocyclic with oxygen as heteroatom (Govori et al 1996 ; Govori et al 2002 ; Stanovnik et al 1993).Coumarin is a chemical sompound (specifically , a benzo-α-pyrone) found in many plants (Lee et al 2002) notably in high concentration in the tonka bean ( Dipteryx odorata), vanilla grass (Anthoxanthum odoratum) , woodruff (Galium odoratum) , mullein (Verbascum spp), and sweet grass (Hierochloe odorata).Coumarine and their derivatives have shown varius biological activities. Their fame has come mainly from their antithrombic, antiinflammatory, vasodilatory , and antiviral activities. Other several coumarin derivatives have antimicrobial properties (Sanghyun ; et al 1996 ; Mohareb et al 2007; Nofal et al 2000), have urged us to synthesize some new coumarin derivatives and to investigate their antibacterial activity against staphylococcus aureus, E.coli and Klebsiella.The antibacterial activity of synthesized compounds is compared with antibacterial activity of streptomycin.
MATERIAL AND METHODS
Experimental Chemistry
Compounds 4-Butylamino-chromen-2-one (1a) , 4-Butylamino-2-oxo-2H-chromene-3-sulfonyl chloride (2a) , 4-Butylamino-2-oxo-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (3a), 4-Butylamino-5-ethyl-2-oxo-7-(N’-phenyl-hydrazino)-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (4a),are synthesized.
The identification of 2H-chromen-2-one derivatives (1a,2a,3a,4a) , is made by using melting point , infrared , 1H NMR , 13C NMR spectra and elemental analysis. Melting point was determinated on a Electrothermal apparatus (Fisher Scientific 2555) in a open capillary tube and are uncorrected.Infrared spectra were recorded in cm-1 for KBr pellts on a FT-IR Shimadzu 8400S spectrophotometer with resolution 4 cm-1 . 1H NMR spectra were recorded on a Bruker UNITY plus-500 ‘NMR 1’ spectrometer using DMSO-d6 as the solvent and TMS as the internal references standard ( σ = 0,00 ppm). Chemical shifts are expressed in δ ppm.Mass spectra were taken on a LKB 9000 mass spectrometer.
Element analysze was performed on a Perikin-Elmer 240 BCHN analyzer.The purity of the compounds (synthesized) was routinely checked by TLC using Merck Kieselgel-60 (F-254) and benzene,toluene,glacial acetic acid (80:10:10)as mobile phase. The spots were exposed in iodine vapour for visualization.
Synthesis of 4-Butylamino-chromen-2-one (1a)
For this synthesis is used as substrat 4-Chloro-chromen-2-one in a 100 ml flask mixed 3 g of 4-Chloro-chromen-2-one with 8ml C2H5OH , equivalent amount Butylamino. The mixture was refluxed at 250 oC for ca. 90 min. The obtained crystals brown are filtred and rinsed with ethanol and dried at room temperature.Recrystallization form absolute ethanol gave a red product of 80% yield, melting point 117oC.
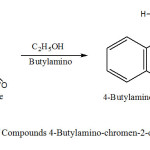 |
Scheme1: Synthesis of Compounds 4-Butylamino-chromen-2-one (1a) Click here to View Scheme |
Synthesis of 4-Butylamino-2-oxo-2H-chromene-3-sulfonyl chloride (2a)
In a 100 ml flask were mixed 2.5 g of 4-Butylamino-chromen-2-one, with 5 ml CH3 CN , 1 ml ClSO3H , 0.3 ml Et3N .
The mixture was refluxed at 80 oC for ca. 1.5 h . The obtained brown crystals are filtred and dried at room temperature . Recrystallization form C2H5OH gave brown crystals product of 70% yield, melting point, 287 oC. (Scheme 2) .
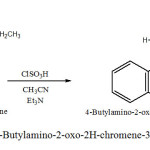 |
Sheme2: Synthesis of 4-Butylamino-2-oxo-2H-chromene-3-sulfonyl chloride (2a) Click here to View Scheme |
Synthesis of 4-Butylamino-2-oxo-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl) -amide (3a)
In a 100 ml flask were mixed 1.5g 4- Butylamino – 2 – oxo – 2H- chromene – 3- sulfonyl chloride with 4 ml Dioxane and 1g aminophenol , 0.2 ml HCl , 0,2 ml Et3N as katalyzer. The mixture was refkuxed at 92 oC in water bath for ca. 2 h .The flask was placed in an ice bath for 1h until yellow crystalline precipitate was formed.
After filtration the product was recrystallized from ethanol .The recrystallizacion from ethanol gave a yellow product at 70% yield, melting.point;180oC. (Scheme 3 ).
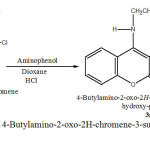 |
Scheme3: Synthesis of 4-Butylamino-2-oxo-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl) -amide (3a) Click here to View Scheme |
Synthesis of 4 – Butylamino – 5 – ethyl -2 – oxo -7 – ( N’ – phenyl – hydrazine )- 2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (4a)
In a 100 ml flask were mixed 1g of 4-Butylamino-2-oxo-2H-chromene-3-sulfonic acid (2-hydroxy-phenyl) – amide , 0.8g phenylhidrazine with 4ml C2H5OH ,0.5ml ClCH2CH3, 0.2 ml Et3N and 0.2 ml HCl. The mixture was refluxed at 95 oC in water bath for ca. 2 h .The obtained red crystals are filtered and rinsed with CH3CN and dried at room temperature.Recrystallization from ethanol gave a red product at 60 % yield , melting point 204 oC. (Scheme 4)
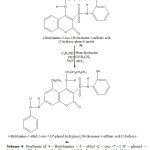 |
Scheme4: Synthesis of 4 – Butylamino – 5 – ethyl -2 – oxo -7 – ( N’ – phenyl – hydrazine )- 2H-chromene-3-sulfonic acid (2-hydroxy-phenyl)-amide (4a) Click here to View Scheme |
Antibacterial activity
The purified synthesized compounds (1a,2a,3a,4a) was subjected to test in vitro its antibacterial activity against three bacterial cultures ; Staphylococcus aureus,E.Coli and Klebsiella. Antibacterial activity of compounds was investigated applying the Kirby-Bayer method 14 or disc method (d=5.5 mm max. capacity 10 μg)
Table 1 Antibacterial activity- Staphylococcus aureus and the comparison with Streptomycine
Inhibition zone (mm)
| Compound | 2mg/ml | 3mg /ml | 5mg/ml |
| 1a | 10 | 13 | 15 |
| 2a | 18 | 20 | 24 |
| 3a | 19 | 21 | 25 |
| 4a | 11 | 13 | 18 |
| Streptomycine | 20 | 20 | 20 10 μg |
Table 2 Antibacterial activity – E.coli and the comparison with Streptomycine
Inhibition zone (mm)
| Compound | 2mg/ml | 3mg /ml | 5mg/ml |
| 1a | 5 | 9 | 14 |
| 2a | 10 | 15 | 21 |
| 3a | 12 | 17 | 23 |
| 4a | 11 | 15 | 20 |
| Streptomycine | 23 | 23 | 23 10 μg |
Table 3 Antibacterial activity – Klebsiella and the comparison with Streptomycine
Inhibition zone (mm)
| Compound | 2mg/ml | 3mg /ml | 5mg/ml |
| 1a | 12 | 19 | 23 |
| 2a | 13 | 18 | 25 |
| 3a | 13 | 19 | 24 |
| 4a | 10 | 17 | 21 |
| Streptomycine | 23 | 23 | 23 10 μg |
Table 4
| Compound | IR (cm-1) | 1H NMR ppm | 13C NMR ppm |
| 1a | 3370 (NH), 3010(C-H) ar, 2962(C-H)alifatic1720(C=O),1570(C=C)ar, 1385(C-O),750(C-H)ar |
δ.0.96 s(3H,CH3) 1.33 d(,4H,2CH2) 1.55-2.0 d(,H,NH-CH2) 2.65 s(,H,NH),7.20-7.60m(,5H,ar) |
δ. 166(C-NH),162(C,COO),150(C-O),121-128(5C,ar)88.9(C=C-H),46.3(C-NH)34.8(C,CH2),20.6(C,CH2)13.7(C,CH3) |
| 2a | 3370(N-H),3008(C-H)ar 2960(CH)alifatic,1740(C=O),1600(C=C)1380(SO2Cl),1285(C-O)720(C-H)ar |
δ.0.96 s(,3H,CH3) 1.33-1.55,d(4H,2CH2)2.65 s(H,NHCH2)3.0 s(H,NH)ar 7.20-7.63m(4H,ar) |
δ.167(C-NH),162(COO),150.8(C-O),121-128(6C,ar)89(C-SO2),46.3(C-NH)34.8(C,CH2),20.6(C,CH2)13.7(C,CH3) |
| 3a | 3400(OH),3300(NH),3265(SO2NH),3009(C-H)ar, 2850 (C-H)al,1730(C=O),1528(C=C) ar,1280(N-H),1275(C-O),1250(C-O),740(C-H)ar | δ. 0.96s(3H,CH3) 1.33-1.55d(4H,2CH2) 2.65s(H,NHCH2) 3.0s(H,NH),4.0s(H,NHSO2)5.0s(H,OH)6.29-6.63m(8H,ar) |
δ.167(C-NH),162(COO),150(C-O),144(C-O),134(C-NH),116-127(9C,ar)46.2(C-NH)20.6(C,CH2)13.7(C,CH3) |
| 4ª | 3387(O-H),3330(N-H) 3270(SO2NH),3010(C-H)ar 2900(C-H)al ,1728(C=O) 1600(C=C)ar,1280(N-H)1270(C-O),750(C-H)ar |
δ.0.96-1.24d(6H,2CH3)1.33-1.55d(4H,2CH2)2.0s(H,NH),2.65s(H,NH)2.59s(H,CH2),4.0t(H,NH)5.0s(H,OH)6.29-7.18m(11H,ar) | δ. 167(C-NH),162(COO),151(C-O),144(C-O),142(C-NH),102-138(17C,ar),89(C-SO2)46.3(C-NH),22.5(C,CH2)13.7(C,CH3),10.5(C,CH3) |
Table-5 Analytical data
| Compd | Yield (%) |
m.p | M.F | Elemental analysis. Calculated (found) (%) | |||||
| C H N O Cl | S | ||||||||
| 1a | 80 | 117oC | C13H15NO2 | 71.8772.00 | 6.967.11 | 6.456.15 | 14.7314.32 | ||
| 2a | 70 | 287oC | C13H14ClNO4S | 49.4550.00 | 4.475.00 | 4.444.11 | 20.2720.00 | 11.2311.00 | 10.159.80 |
| 3a | 70 | 180oC | C19H20N2O5S | 58.7560.00 | 5.194.90 | 7.217.10 | 20.5919.92 | 8.208.00 | |
| 4a | 60 | 204oC | C27H30N4O5S | 62.0561.50 | 5.795.20 | 10.7210.0 | 15.3115.00 | 6.146.00 | |
CONCLUSION
From the results the follow in conclusion were drawn:The study provides the first evidence that compounds (1a,2a,3a,4a) obviously inhibit the growth of Staphyllococcus auerus , E.coli and Klebsiella.
The compounds (1a,2a,3a,4a) compared with the antibacterial activity of Streptomycine in S.aureus , and Klebsiella.
The chemical structures of synthesizen compounds were determined according to extensive NMR experiments and published data.
ACKNOWLEDGEMENTS
The authors thank Prof.Branko Stanovnik,University of Ljubljana and its laboratory staff for 1H NMR spectrum and elemental analyses.
REFERENCES
- S.Govori,V.Kalaj,V.Rapic,L.Kalaj and S.Dakovic,Heterocycel.Commun., 2002; 8,129.
- B.Stanovnik, H.Susachitzky and E.F.Scriven, Progress in Heterocyclic Chemistry, Pergamon Press ,Oxford , 1993; 5,pp.75-146.
- S.H.Lee, D.-S.Shin, J.-S,Kim, K.-B. Oh and S.S.Kan, Arch. Pharm. Res, 2003; 26.
- K.B.Vyas, K.S.Nimavat, G.R.Jani and M.V. Hathi, Orbital, 2009; 1, 183.
- A.Z.Abyshev, V.A.Gimdein, E.V. Semenov, E.M, E.MAgev, A.A. Abdulla – Zade and A.B.Gueseinov,Pharm.Chem.J., 2206; 40.607.
- M.D.Aytemir , R.C.Hider , D.D.Erol , M.Ozalp and M.Ekizoglu .Turk.J.chem., 2003; 27 : 445.
- M.M.El.Saghier,M.B.Naili,B.Kh.Rammash, N.A.Saleh and K.M.Kreddan, Arkivoc, 2007; 83.
- Z.M.Nofal ,M.El-Zahar and S.Abd El Karim,Molecules, 2000; 5: 99.
- Chaluvaraju KC and Ishwarbhat K.Asian ,J Chem 2008; 20, 4335.
- Rajan Ra Kali , Jubie S,Grworamma B, and Suresh B,Asian J Chem 2008: 20: 5289.
- Ali Mohammed Ashraf and Sharayar Mohammed . Boorg Med Chem. Lett 2009; 17: 3314.
- Nofal ZM, El-Zahar M, Abd El-Karim S, Novel coumarin derivatives with expected biological activity, Molecules. 2000; 5: 99-113.
- Vyas KB,NimavatKS,Jani GR,Hathi MV, Synthesis and microbial activity of coumarin derivatives metal complex: An in vitro evaluation.Orbital, 2009; 1: 183-192.

This work is licensed under a Creative Commons Attribution 4.0 International License.









