Synthetic Approaches to (R)-Cyclohex-2-Enol
Nilesh Zaware, Michael Ohlmeyer
Department of Structural and Chemical Biology, Mount Sinai School of Medicine,
1425 Madison ave, New York, NY10029.
DOI : http://dx.doi.org/10.13005/ojc/300102
Article Received on : January 25, 2014
Article Accepted on : March 06, 2014
Article Published : 13 Mar 2014
(R)-Cyclohexenol is a valuable building block in organic synthesis. This mini-review provides methods for synthesis of (R)-cyclohexenol from commercially available reactants. Only reactions with yields in excess of 80% are discussed (ee’s range from 99% to 26%). The asymmetric synthesis methods include enantioselecive deprotonation of cyclohexene oxide by chiral lithium amides, asymmetric hydrosilylation of 2-cyclohexen-1-one with chiral catalyst followed by hydrolysis, and enantioselective hydroboration of 1,3-cyclohexadiene with chiral dialkylboranes.
KEYWORDS:(R)-cyclohexenol; asymmetric synthesis; chiral Li-amides
Download this article as:| Copy the following to cite this article: Zaware N, Ohlmeyer M. M.Synthetic Approaches to (R)-Cyclohex-2-Enol . Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Zaware N, Ohlmeyer M. M.Synthetic Approaches to (R)-Cyclohex-2-Enol. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2392 |
Introduction
Asymmetric synthesis starting from achiral reactants to produce synthetically useful chiral compounds is an attractive and established branch of synthetic organic chemistry. (R)-cyclohexenol 1 serves as a versatile chiral precursor for synthesis of natural products and complex medicinally active compounds like (+) Daphmandin E 2,(1) and antiarrhythmic aminohydroisoquinocarbazole RS-2135 3 (2) respectively (Figure 1)
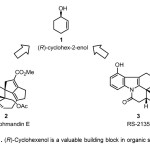 |
Figure 1. (R)-Cyclohexenol is a valuable building block in organic synthesis Click here to View Figure |
Figure 1. (R)-Cyclohexenol is a valuable building block in organic synthesis A scifinder scholar® search of methods to synthesize (R)-cyclohexenol in February 2014 resulted in 129 hits. This mini-review discusses methods from these 129 results wherein the isolated yield for the target from commercially available reactants was in excess of 80% and the ee ranged from 99% to 26%. This mini-review is intended to present synthetic chemists with a guide for synthesis of (R)-cyclohexenol and its derivatives.
SYNTHETIC STRATEGIES TO (R)-CYCLOHEXENOL
FROM CYCLOHEXENE OXIDE
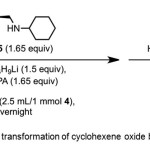 |
Scheme 1. Asymmetric transformation of cyclohexene oxide by catalyst 5. Click here to View Scheme |
Asami et al.(3) report conversion of cyclohexene oxide 4 to 1 (80% yield, 78% ee) using chiral catalyst – cyclohexyl[(S)-1-ethylpyrrolidin-2-yl]methylamine 5 from (Scheme 1). The mechanism involves enantioselective deprotonation of symmetrical epoxide 4 using the chiral lithium amide prepared from n-butyl lithium and 5. HMPA is used as an additive. It has been suggested that additives inhibit the formation of reactive but unselective aggregates of chiral Li-amides.(4-7)
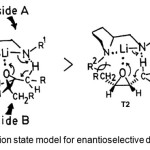 |
Figure 2. Transtion state model for enantioselective deprotonation by 5. Click here to View Figure |
As lithium amide induced transformation of epoxides to allylic alcohols I supposed to proceed in a cyclic concerted manner,(8) Asami et al.(3) presume the transition states’ (TSs’) as shown in figure 2 to account for the stereoselectivity of the reaction. As indicated in transition state T1, epoxide approaches the lithium amide from the less hindered side in such a way that the steric repulsion can be avoided. Thus T1 is favored over T2, and the alcohol with R-configuration is obtained.
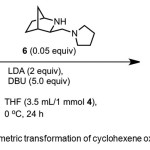 |
Scheme 2. Asymmetric transformation of cyclohexene oxide by catalyst 6. Click here to View Scheme |
Sodergren et al.(9) report conversion of cyclohexene oxide 4 to 1 (91% yield, 96% ee) using chiral catalyst – 3-aminomethyl-2-azabicyclo[2.2.1]heptane 6 (Scheme 2). DBU is used as an additive. The authors reasoned that Li-amide with a more rigid back bone would adopt a more well-ordered TS in the deprotonation reaction to afford higher asymmetric induction as the result of more strict discrimination between the enantiotopic protons in 4.
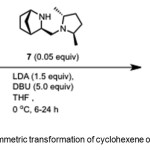 |
Scheme 3. Asymmetric transformation of cyclohexene oxide by catalyst 7. Click here to View Scheme |
Bertilsson et al.(10) report conversion of cyclohexene oxide 4 to 1 (95% yield, 99% ee) using chiral catalyst – (1R,3R,4S)-3-(((2R,5R)-2,5-dimethylpyrrolidin-1-yl)methyl)-2-azabicyclo[2.2.1]hept-5-ene 7 (Scheme 3).
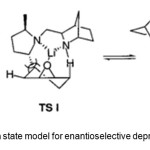 |
Figure 3. Transtion state model for enantioselective deprotonation by 7. Click here to View Figure |
As indicated in the TS model (Figure 3), the (2R,5R)-dimethyl groups do not interfere with the favored TS II, whereas the unfavored pathway is effectively blocked by the steric repulsion between the (2R)-methyl group and the cis-g -proton of the epoxide.
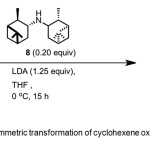 |
Scheme 4. Asymmetric transformation of cyclohexene oxide by catalyst 8. Click here to View Scheme |
Malhotra(11) reports conversion of cyclohexene oxide 4 to 1 (82% yield, 95% ee) using chiral catalyst – C2-symmetric (−)-N,N-diisopinocampheyl- amine (DIPAM) 8 (Scheme 4). No additive was used in the reaction.
FROM 2-CYCLOHEXEN-1-ONE
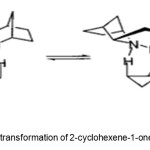 |
Scheme 5. Asymmetric transformation of 2-cyclohexene-1-one by ZnEt2/pybox system. Click here to View Scheme |
Junge et al.(12) report conversion of 2-cyclohexen-1-one 9 to 1 (88% yield, 26% ee) by asymmetric hydrosilylation with a combination of ZnEt2, chiral 2,6-bis((R)-4-phenyl-4,5-dihydrooxazol-2-yl)-pyridine (pybox) catalyst 10, and polymethylhydrosiloxane (PMHS), followed by hydrolysis to the alcohol (Scheme 5).
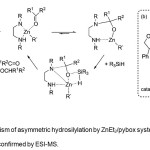 |
Figure 4. (a) Mechanism of asymmetric hydrosilylation by ZnEt2/pybox system. (b) Catalytically active species 11 as confirmed by ESI-MS. Click here to View Figure |
The proposed mechanism of the asymmetric hydrosilylation process (Figure 4a) by Mimoun et al.(13,14) was confirmed by Junge et al.(12) by confirming the presence of 11 (Figure 4b) by ESI-MS studies.
FROM 1,3-CYCLOHEXADIENE
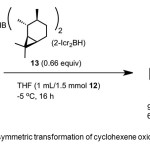 |
Scheme 6. Asymmetric transformation of cyclohexene oxide by catalyst 8. Click here to View Scheme |
Zaidlewicz et al.(15) report conversion of 1,3-cyclohexadiene 12 to 1 (94% yield, 68% ee) using chiral catalyst – di-(2-isocaranyl)borane (2-Icr2-BH) 13 (Scheme 6). Mechanism involves the enantioselective hydroboration of 12 in presence of bulky dialkylboranes.
CONCLUSION
In conclusion, we present here enantioselective approaches to (R)-cyclohexanol using commercially available reactants such as cyclohexene oxide, 1,2-cyclohexenone, and 1,3-cyclohexadiene. Reactions enantioselecive deprotonation of cyclohexene oxide by chiral lithium amides, asymmetric hydrosilylation of 2-cyclohexen-1-one with chiral catalyst followed by hydrolysis, and enantioselective hydroboration of 1,3-cyclohexadiene with chiral dialkylboranes. The yields of the aforementioned methods range from 95 to 80% and the ee’s range from 99 to 26%.
ACKNOWLEDGEMENTS
Dual Therapeutics LLC, and Grant # 0249-1924 from BioMotiv, LLC, are gratefully acknowledged.
REFERENCES
- Weiss, M. E.; Carreira, E. M. Angewandte Chemie (International ed. in English), 50, 11501 (2011).
- Fukazawa, T.; Shimoji, Y.; Hashimoto, T. Tetrahedron-Asymmetry, 7, 1649 (1996).
- Asami, M.; Kirihara, H. Chem Lett., 389 (1987).
- Hodgson, D. M.; Gibbs, A. R.; Lee, G. P. Tetrahedron, 52, 14361 (1987).
- Asami, M.; Takahashi, J.; Inoue, S. Tetrahedron: Asymmetry, 5, 1649 (1994).
- Asami, M. Tetrahedron Letters, 26, 5803 (1985).
- Asami, M. Bull. Chem. Soc. Jpn., 63, 721 (1990).
- Thummel, R. P.; Rickborn, B. J. Am. Chem. Soc., 92, 2064 (1970).
- Sodergren, M. J.; Andersson, P. G. J. Am. Chem. Soc., 120, 10760 (1998).
- Bertilson, S. K.; Sodergren, M. J.; Andersson, P. G. J. Org. Chem., 67, 1567 (2002).
- Malhotra, S. V. Tetrahedron-Asymmetry, 14, 645 (2003).
- Junge, K.; Moller, K.; Wendt, B.; Das, S.; Gordes, D.; Thurow, K.; Beller, M. Chem-Asian J., 7, 314 (2012).
- Mimoun, H. J. Org. Chem., 64, 2582 (1999).
- Mimoun, H.; de Saint Laumer, J. Y.; Giannini, L.; Scopelliti, R.; Floriani, C. J. Am. Chem. Soc. 121, 6158 (1999).
- Zaidlewicz, M.; Walasek, Z. Pol J Chem., 68, 2489 (1994).

This work is licensed under a Creative Commons Attribution 4.0 International License.









