Nerita chameleon as Biomonitoring Agent for Pb, Cd, Cu and Zn in Malaysian Intertidal Rocky Shore Environment
Mohd Fuad Miskon1, Noor Azhar Mohd Shazili2, Faridah Mohamad3, Kamaruzzaman Yunus*1
1Kuliyyah of Science, International Islamic University Malaysia, Bandar Indera Mahkota, 25200 Kuantan, Pahang, Malaysia 2Faculty of Maritime Studies and Marine Science, Universiti Malaysia Terengganu, 21030, Kuala Terengganu, Malaysia 3Department of Biological Science, Faculty of Science and Technology, Universiti Malaysia Terengganu, 21030, Kuala Terengganu, Malaysia Corresponding author: E-mail address: kama@iium.edu.my
DOI : http://dx.doi.org/10.13005/ojc/310249
Article Received on :
Article Accepted on :
Article Published : 02 Jun 2015
Pb, Cd, Cu and Zn in the soft tissue of Nerita chameleon from particular rocky shore sites along the east coast of Peninsular Malaysia were investigated. Samples were measured using ICP-MS with standard configuration. The metal accumulation patterns indicate consistent enrichment of essential metals. Locations with relatively high concentrations of the contaminant metals Pb, Cd and Cu are related to their close proximity to industrial activities and urban sites. Comparison with maximum permissible limits of toxic metals in food indicated the values were well within safety levels.
KEYWORDS:Nerita chameleon; east coast of Peninsular Malaysia; heavy metals; food safety level
Download this article as:| Copy the following to cite this article: Miskon M. F, Shazili N. A. M, Mohamad F, Yunus K. Nerita chameleon as Biomonitoring Agent for Pb, Cd, Cu and Zn in Malaysian Intertidal Rocky Shore Environment. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Miskon M. F, Shazili N. A. M, Mohamad F, Yunus K. Nerita chameleon as Biomonitoring Agent for Pb, Cd, Cu and Zn in Malaysian Intertidal Rocky Shore Environment. Available from: http://www.orientjchem.org/?p=9000 |
Introduction
Gastropods from the family Neritidae (Rafinesque, 1815) lives in marine, brackish, and freshwater systems. This colourful round snail, N. chameleon is commonly seen in rocky shore and feed on algae. Along the coast, these herbivores usually inhabit the middle to upper intertidal zones and are known to be gregarious (Tan and Clements, 2008). It is collected as food by coastal dwellers as well as for its shell for the shell trade. Studies on the bioaccumulation of heavy metals by Nerita sp. in various parts of the world have been well documented. Research by Abdullah and Moustafa (2001) revealed that N. saxtilis is capable of bioaccumulating Pb and Cd. Blackmore (2001) reported that N. albicilla is capable of accumulating Cu and Zn due to the physiological requirements for these elements. In Malaysia, study by Kanakaraju and Anuar (2009) suggested N. lineata as a potential bioindicator of heavy metals in the intertidal areas of Peninsular Malaysia and Sarawak. Only a little studies that using N. chameleon as bioindicator in trace metals monitoring has been found.
Various metal-accumulating bivalve and gastropod species show a high presence and abundance and play an important role as bioindicators for pollution in global monitoring programs throughout the world (Wang et al., 2005). Molluscs exhibit greater spatial sensitivity, thus, are the most reliable tool for identifying sources of bioavailable contamination unlike sediments (Goldberg et al., 1978; Hamed and Emara, 2006). According to Shazili et al. (2006), the information on the levels of heavy metals pollution in the Malaysian aquatic environment is scarce and limited to a small number of studies as well as specific limitation on certain elements. Most of the coastal resources, agriculture and economic activities and human population are concentrated on the west coast of Peninsular Malaysia (Abdullah et al., 1999). Only a minor research have contribute data for the east coast of peninsular Malaysia on the shores of the South China Sea.
The present study is to provide a baseline data for the distribution of Pb, Cd, Cu and Zn in soft tissue of N. chameleon collected from polluted and unpolluted environments. The use gastropod as bioindicator is also related to their importance in human consumption. The result directly determines the bioavailability of polluted elements within targeted species and gives an indication of the extent of pollution by these elements. Comparison with permissible limits in Malaysia and other countries as well, provide an insight in their safety levels for human.
Materials and Methods
15 sampling sites that includes pristine, recreational sites and proximity of industrial and urbanized areas (Figure 1) were done. Various types of enviroenment were set as to provide a wider range of insight on bioaccumulation patterns related to environmental status. Only sites having abundant numbers of N. chameleon population on natural rocky structures were taken. Relatively same size of N. chameleon were hand collected during low tide. Samples were allowed to clean their gut by depurate them in their habitat water with aeration for 24 hours. Depurated samples were kept in plastic bags, sealed, labeled and stored at 4 – 6°C and being transported to the laboratory. Samples were rinsed with running Mili-Q water (18.2 Ω) to remove sediment and salt particles prior to store frozen. Once allometric parameter measurement and tissue extraction has been done, extracted soft tissues were freeze dried before being weighed again in order to estimate the water content and conversion factor. Freeze-dried samples were then milled to a homogenous powder and retained at room temperature until heavy metals measurement.
The analytical method was based on Shazili et al. (2009) with little modification. Analysis was done using Inductive Coupled Plasma Mass Spectrometer (ICP-MS) Perkin Elmer Elan 9000 using standard configuration. Results were blank corrected and expressed as µg g-1 dry weight. Glassware used was immersed in 10% HNO3 solution in advance for contamination avoidance. The quality of method used was confirmed in a separate comparative analysis using a standard reference material, Lobster Hepatopancreas TORT-2. Recoveries were as follows: 113.57% – Pb, 97.31% – Cd, 85.56% – Cu and 86.54% – Zn.
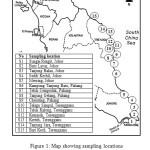 |
Figure1: Map showing sampling locations on the east coast of Peninsular Malaysia |
SPSS (Version 17.0, SPSS Inc., Chicago, USA) was used for determining statistical data including descriptive statistics (mean and standard deviation in variable list display order, one-way analysis of variance (ANOVA), Pearson’s correlation coefficient and hierarchical cluster analysis. Excel 2010 (Microsoft Corporation, Washington, USA) was applied for graphic assignment. Hierarchical cluster analysis was employed for assessment of homogenous groups or location for the impact of the concentration observed.
Results and Discussion
Table 1 shows the percentage water contents and conversion factor of N. chameleon while concentrations of Pb, Cd, Cu and Zn in soft tissue are shown in Figure 2. Significant differences (p<0.05) among concentrations of all studied metals between locations were found, except for Cd. Soft tissue from Sg. Rengit evidently contained highest concentrations of Cu. The highest concentration of Pb, Cd and Zn were found at Kg. Tg. Batu, Mersing and Chendering, respectively. The mean values of Cd and Pb from Mersing and Kg. Tg. Batu, respectively, were found to be more than two-fold higher than other locations.
Table1: Means of wet weight, dry weight, water content (%) and conversion factor (CF) of Nerita chameleon from the east coast of Peninsular Malaysia
|
No |
Location |
Wet weight (g) |
Dry weight (g) |
Water content (%) |
CF |
|
1 |
Sg. Rengit |
0.48±0.27 |
0.10±0.05 |
78.70±3.10 |
0.21 |
|
2 |
Bt. Layar |
0.39±0.30 |
0.08±0.05 |
77.62±3.96 |
0.21 |
|
3 |
Tg. Balau |
0.59±0.30 |
0.10±0.04 |
82.16±4.14 |
0.17 |
|
4 |
Sedili Kecil |
0.36±0.19 |
0.07±0.03 |
79.36±3.32 |
0.19 |
|
5 |
Mersing |
0.51±0.18 |
0.10±0.04 |
79.15±2.14 |
0.20 |
|
6 |
Kg. Tg. Batu |
0.24±0.06 |
0.07±0.02 |
72.55±3.15 |
0.29 |
|
7 |
Tlk. Cempedak |
0.36±0.12 |
0.08±0.03 |
76.54±3.20 |
0.22 |
|
8 |
Tg. Gelang |
0.35±0.07 |
0.08±0.02 |
76.72±1.96 |
0.23 |
|
9 |
Cherating |
0.41±0.18 |
0.10±0.04 |
77.70±2.66 |
0.24 |
|
10 |
Telaga Simpul |
0.36±0.14 |
0.07±0.03 |
80.16±2.20 |
0.19 |
|
11 |
Tlk. Kalong |
0.17±0.04 |
0.04±0.01 |
75.74±3.33 |
0.24 |
|
12 |
Kemasik |
0.37±0.13 |
0.10±0.03 |
73.32±5.56 |
0.27 |
|
13 |
Kerteh |
0.74±0.25 |
0.16±0.05 |
78.70±1.40 |
0.22 |
|
14 |
Tg. Jara |
0.44±0.22 |
0.11±0.06 |
75.82±3.05 |
0.25 |
|
15 |
Chendering |
0.43±0.23 |
0.10±0.05 |
77.31±2.46 |
0.23 |
The cluster analysis (Figure 3) showed that less numbers of homogenous groups can be found for Cd and Pb. Interestingly, Kg. Tg. Batu was locate differently from the others in a single group for Pb. Other homogenous groups of locations were variable and thus, difficult to classify and do not show any particular trend. As a whole, major differences of metal concentrations among locations were generally found at Sg. Rengit, Tg. Balau and Kg. Tg. Batu.
Kg. Tg. Batu site may be considered a pristine area but pollution at this area is assumed to derive from the organic wastes discharged from a massive shrimp culture complex nearby at the north. Heavy metal such as Pb contamination has been found to originate from aquaculture activities (Yap et al., 2002) and organic wastes discharged from fish farms were found to have impacts on the water quality around fish culture zones (Wu et al., 1994; Yap et al., 2003).
 |
Figure2: Pb, Cd, Cu and Zn distribution (µg g-1 dry weight) in soft tissue of Nerita chameleon from 15 sampling locations along the east coast of Peninsular Malaysia Click here to View figure |
A comparison of metals concentrations among locations lead to the conclusion that high concentrations in all studied species are generally found at Sg. Rengit, Mersing and Kg. Tg. Batu. High concentrations of metals in Sg. Rengit is expected as this location lies at the vicinity of urbanized area and medium-sized fisheries port that runs heavy traffic of fishing boats. Besides, this location lies at the river mouth that channels effluents from the nearby urbanized area directly to sea. Site at Mersing was next to ferry jetty for Tioman Island trip, thus metal elevation here can be expected.
 |
Figure3: Hierarchical cluster analysis of Cd, Pb, Cu, and Zn from 15 sampling locations of Nerita chameleon from the east coast of Peninsular Malaysia Click here to View figure |
Table 2 shows the order of metal concentrations in the soft tissues of N. chameleon from 15 sampling locations along the east coast of Peninsular Malaysia. The metal concentrations were observed to be in decreasing order in the soft tissue of N. chameleon which can be generally presented as Zn > Cu > Pb > Cd. Essential metals (Zn and Cu) showed highest accumulation. Non-essential metals (Pb and Cd) were found as the lowest metals accumulated.
In respect to accumulation of Zn and Cu in N. chameleon, Zn concentrations, however, are always higher than Cu. Zn and Cu are emphasized as they evidently proved to being essential in living organisms in the functioning of metabolic processes and associated with many enzymatic proteins functions (White and Rainbow, 1995; Amiard et al, 2008). Zata (1984, 1985) reported that each functional unit of haemocyanin is capable of binding two molecules of Cu and four of Zn. This let to conclude that many potential binding sites lies for Zn compare with Cu, thus distinguish the concentrations of both metals within gastropods.
Table 2: Decreasing order of metal occurrences (µg g-1 dry weight) in soft tissue of Nerita chameleon from 15 sampling locations along the east coast of Peninsular Malaysia
| Site |
Pattern |
| Sg. Rengit |
Cu > Zn > Pb > Cd |
| Bt. Layar |
Zn > Cu > Pb > Cd |
| Tg. Balau |
Zn > Cu > Cd > Pb |
| Sedili Kecil |
Zn > Cu > Pb > Cd |
| Mersing |
Zn > Cu > Cd > Pb |
| Kg. Tg. Batu |
Zn > Cu > Pb > Cd |
| Tlk. Cempedak |
Zn > Cu > Pb > Cd |
| Tg. Gelang |
Zn > Cu > Pb > Cd |
| Cherating |
Zn > Cu > Pb > Cd |
| Telaga Simpul |
Zn > Cu > Pb > Cd |
| Tlk. Kalong |
Zn > Cu > Pb > Cd |
| Kemasik |
Zn > Cu > Pb > Cd |
| Kerteh |
Zn > Cu > Pb > Cd |
| Tg, Jara |
Zn > Cu > Cd > Pb |
| Bari Kecil |
Zn > Cu > Cd > Pb |
A specific observation into Order taxonomy classification reveals that N. chameleon in this study falls into archeogastropod. Previous research by Blackmore (2001) has confirmed that the archaeogastropod had low Cu body concentration in their enzymatic requirement; therefore, explain the lower accumulation of Cu within N. chameleon. It was also in agreement with that finding that shows the same pattern of low accumulation of Zn and Cu in the archaeogastropod (60.4 – 71.1 µg g-1 dry weightsfor Zn and 3.96 – 9.84 µg g-1 dry weights for Cu).
It is clear that the essential metals like Zn and Cu are among the highest accumulated metals. This is expected as in soft tissue, these metals has been acknowledged as biologically essential and needed for metabolism (Joleey et al., 2004; Amin et al., 2006), play an important role as cofactors in enzymatic processes and are a functional part of the respiratory protein haemocyanin (Rainbow, 2002). Hence, these metals cannot be immediately excreted or detoxified as they are required to play roles in metabolism. Non-essential metals like Pb and Cd are among less accumulated metals as they are not needed in metabolism and would have no required minimum concentration. However, they are taken up by all organisms in proportion to the degree of environmental contamination levels.
Table3: Maximum permissible limits on heavy metals (in µg g-1) for food safety set by different countries
|
Weight basis |
Pb |
Cd |
Cu |
Zn |
|
| Food category not specified | |||||
| Brazil |
Dry |
10a |
5a |
150a |
250a |
| Thailand |
Dry |
6.67b |
133b |
667b |
|
| Shellfish molluscs | |||||
| European Community |
Wet |
1.5c |
1c |
||
| Hong Kong |
Wet |
6d |
2d |
||
| Australia |
Wet |
2e |
2e |
30f |
|
| USA |
Wet |
1.7g |
3.7g |
||
| Malaysia |
Wet |
2h |
1h |
30h |
100h |
| This study |
Wet |
0.77 |
0.55 |
8.18 |
14.34 |
|
Dry |
3.32 |
2.52 |
36.80 |
64.10 |
|
a Brazilian Ministry of Health (ABIA, 1991)
b Ministry of Public Health, Thailand (MPHT, 1986)
c European Community (EC, 2006)
d Hong Kong Environmental Protection Department (HKEPD, 1997)
e Food standards Australia New Zealand Authority (FSANZ, 1996)
f Australian Government, 2006
g Food and Drug Administration of the United States (USFDA, 1990)
h Malaysian Food Regulation Fourteen Schedule (1985)
*Values that exceed the permissible limits set by Malaysian Food Regulation Fourteen Schedule
To safeguard public health, maximum acceptable concentrations of toxic contaminants have been established in various countries, including Malaysia. Most of safety levels established was expressed on a wet weight basis. Therefore, metal concentrations for each species in this study have been calculated into wet weight basis using conversion factor (Table 1) for comparison with permissible limits set by Malaysia and other different countries (Table 3). All heavy metals in N. chameleon recorded in the present study are below the permissible limits either in Malaysia or from other countries as well.
Conclusion
The present work has provided a synoptic survey of Pb, Cd, Cu and Zn distribution in N. chameleon in rocky shore areas along the east coast of Peninsular Malaysia. This species possessed high potential as cosmopolitan bioindicators for Pb, Cd, Cu and Zn as they have the necessary prerequisites of bioindicators: they are easy to identify and to sample, are available throughout the year, and are widely abundant in almost all rocky shore areas along the east coast of Peninsular Malaysia. From the metal comparison permissible data, it can be concluded that N. chameleon is still safe for human consumption.
References
- Tan, S. K.; Clements, R. Taxonomy and distribution of the Neritidae (mollusca: Gastropoda) in Singapore. Zoological studies. 2008, 47(4), 481-494.
- Abdullah, A. T.; Moustafa, M. A. Accumulation of lead and cadmium in the marine prosobranch Nerita saxtilis, chemical analysis, light and electron microscopy. Environmental Pollution. 2001, 116, 185-191.
- Blackmore, G. Interspecific variation in heavy metal body concentrations in Hong Kong marine invertebrates. Environmental Pollution. 2001, 114(3), 303-311.
- Kanakaraju, D.; Anuar, A. Accumulation and depuration of lead and chromium using Nerita lineata. World Applied Science Journal. 2009, 6(9), 1205-1208.
- Wang, Y.; Liang, L.; Shi, J.; Jiang, G. Study on the contamination of heavy metals and their correlations in mollusks collected from coastal sites along the Chinese Bohai Sea. Environment International. 2005, 31, 1103-1113.
- Goldberg, E. D. The mussel watch – A first step in global marine monitoring. Marine Pollution Bulletin. 1978, 6(7), 111-114.
- Hamed, M. A.; Emara, A. M. Marine molluscs as biomonitors as biomonitors for heavy metal levels in the Gulf of Suez, Red Sea. Journal of Marine Systems. 2006, 60(3-4), 220-234.
- Shazili, N. A. M.; Yunus, K.; Ahmad, A. S.; Abdullah, N.; Rashid, M. K. A. Heavy metal pollution status in the Malaysian aquatic environment. Aquatic Ecosystem Health and Management. 2006, 9, 137-145.
- Abdullah, A. R.; Tahir, N. M.; Tong, S. L.; Hoque, T. M.; Sulaiman, A. H. The GEF/UNDP/IMO Malacca Straits Demonstration Project: Sources of Pollution. Marine Pollution Bulletin. 1999, 39, 229-233.
- Shazili, N. A. M., Azlisham, M.; Vedamanikam, V. J. Concentrations of cadmium, manganese, copper, zinc and lead in the tissues of the oyster (Crassostrea iradalei) obtained from Setiu Lagoon, Terengganu, Malaysia. Toxicological & Environmental Chemistry. 2009, 91(2), 251-258.
- Yap, C. K.; Ismail, A.; Tan, S. G.; Omar, H. Concentrations of Cu and Pb in the offshore and intertidal sediment of the west coast of Peninsular Malaysia. Environment International. 2002, 28, 467-479.
- Wu, R. S. S.; Mackay, D. W.; Lau, T. C.; Yam, V. Impact of marine fish farming on water quality and bottom sediment: A case study in the sub-tropical environment. Marine Environmental Research. 1994, 38, 115-145.
- Yap, C. K.; Ismail, A.; Tan, S. G.; Rahim, A. Can the shell of the green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia be a potential biomonitoring material for Cd, Pb and Zn? Field and laboratory studies. Estuarine, Coastal and Shelf Science. 2003, 57, 623-630.
- White, S. L.; Rainbow, P. S. On the metabolic requirements for copper and zinc in molluscs and crustaceans. Marine Environmental Research. 1985, 16, 215-229.
- Amiard, J. C.; Triquet, C. A.; Charbonnier, L.; Mesnil, A.; Rainbow, P. S.; Wang, W. Bioaccessibility of essential and non-essential metals in commercial shellfish from Western Europe and Asia. Food and Chemical Toxicology. 2008, 46(6), 2010-2022.
- Zata, P., 1984. Zinc transport in the haemolyph of Carcinus maenas (Crustacea, Decapoda). Marine Biological Association of the United Kingdom, 84: 801-807.
- Zata, P., 1985. Interactions between Zn2+, Co2+, Mn2+ with haemocyanin from Carcinus maenus. Cahiers Marine Biology, 26: 241-249.
- Jolley, D. F.; Maher, W. A.; Kyd, J. Selenium accumulation in the cockle Anadara trapezia. Environmental Pollution. 2004, 132, 203-212.
- Amin, B.; Ismail, A.; Arshad, A.; Yap, C. K.; Kamarudin, M. S. A comparative study of heavy metal concentrations Nerita lineata from the intertidal zone between Dumai, Indonesia and Johor, Malaysia. Journal of Coastal Development. 2006, 10, 19-32.
- Rainbow, P. S. Trace metal concentrations in aquatic invertebrates: why and so what?. Environmental Pollution. 2002, 120(3), 497-507.
- ABIA (Associacao Brasileira das Industrias de Alimentacao), Brazilian Food Industries Association. 1991.
- MPHT (Ministry of Public Health and Medical Research Council). Residues in Food. 1986, 103, 1123-1124.
- CE (Commission Europeénne). Regulation (CE) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union. 2006.
- HKEPD (Hong Kong Environmental Protection Department). Marine water quality in Hong Kong in 1997. Government Printer Hong Kong. 1997.
- FSANZ (Food Standards Australia New Zealand Authority). 1996.
- Australian Government. The MRL Standard. 2006.
- USFDA (Food and Drug Administration of the United States). US Food and Drug Administration Shellfish Sanitation Branch, Washington, DC. 1990.
- Malaysian Food Regulation. Fourteenth Schedule. Malaysian law on food and drugs. Malaysian Law Publishers. 1985.

This work is licensed under a Creative Commons Attribution 4.0 International License.









