Anti-cancer properties of green Tea Probed viaquantum mechanics calculations
Azin Chitsazan
Department of Chemistry, Science and research branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/310147
Article Received on :
Article Accepted on :
Article Published : 13 Mar 2015
Tea, from the plant camellia sinensis, is consumed in different parts of the world as green, black or oolong tea. Among all of these, however, the most significant effects on human health have been observed with the consumption of green tea. Green tea contains polyphenols, which include flavanols, flavandiols, flavonoids, and phenolic acids. Most of the green tea polyphenols (GTPs) are flavonols, commonly known as catechins. There are four kinds of catechins mainly find in green tea: epicatechin, epigallocatechin, epicatechin-3-gallate, and EGCG. Green tea catechins have demonstrated significant antioxidant, anticarcinogenic, anti-inflammatory, thermogenic, probiotic, and antimicrobial properties in numerous human, animal, and in vitro studies. In the present study, four type catechins of green tea were studied. For each catechin ab initio method was employed for calculations and related parameters were computed.
KEYWORDS:calculation; green tea; catechin; polyphenol; EGCG; antioxidant; anticarcinogenic
Download this article as:| Copy the following to cite this article: Chitsazan A. Anti-cancer properties of green Tea Probed viaquantum mechanics calculations. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Chitsazan A. Anti-cancer properties of green Tea Probed viaquantum mechanics calculations. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7654 |
Introduction
Tea is the most consumed drink in the world after water. Unlike black and oolong tea, green tea production does not involve oxidation of young tea leaves [1-3].
Green tea is produced from steaming fresh leaves at high temperatures, thereby inactivating the oxidizing enzymes and leaving the polyphenol content intact [4-8].
The polyphenols found in tea are more commonly known as flavanolsarcatechins, and comprise 30-40 percent of extractable solids of dried green tea leaves. The main catechins in green tea are epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG), with the latter being the highest in concentration [9-13].
Green tea polyphenols have demonstrated significant antioxidant, anticarcinogenic, anti-inflammatory, thermogenic, probitic, and antimicrobial properties in numerous human, animal, and in vitro studies [14-18].
The anticarcinogenic properties of green tea polyphenols, mainly EGCG, are likely a result of inhibition of tumor initiation and promotion, induction of apoptosis, and inhibition of cell replication rates, thus retarding the growth and development of neoplasms [19-22]. Green tea polyphenols antioxidant potential is directly related to the combination of aromatic rings and hydroxyl groups that make up their structure, and is a result of binding and neutralization of free radicals by the hydroxyl groups [23-27].
The potential health effects of catechins depend not only on the amount consumed but on their bioavailability which appears to be very variable. In order to known the catechin bio availability and metabolism, it is necessary to evaluate their biological activity within target tissues [28-36].
Green tea is considered a dietary source of antioxidant nutrients: green tea is rich in polyphenols(catechins and gallic acid, particulary), but is also contains carotenoids, tocopherols, ascorbic acid (vitamin C), minerals such as Cr, Mn, Se or Zn, amd certain phytochemical compounds. These compounds could increase the green tea polyphenols (GTP) antioxidant potential. The antioxidant capacity of GTP has been assessed by several methods [37-41].
For example, Cao et al. [42] using the oxygen radical absorbance capacity (ORAC) assay found that green tea has a much higher antioxidant activity against peroxyl radicals than vegetables such as garlic, kale, spinach and Brussels sprouts. Langley-Evans [43]found that the total antioxidant capacity of green tea is more potent than that of black tea. Saffari and Sadrzadeh[44] investigated the antioxidant capacity of EGCG using erythrocyte membrane- bound [45-51].
ATPases as model, and the results indicated that EGCG is a powerful antioxidant that is capable of protecting erythrocyte membrane-bound ATPases against oxidative stress. Several studies have shown that EGCG can act in vitroas an antioxidant by trapping proxyl radicals and inhibiting lipid peroxidation [44,52]. However, the antioxidant capacity of catechins determined in vitrois dependent upon the type of assay and it does not reflect factors such as bio availability and metabolism [53-59].
Methods and Calculations
Computational methods are well placed to a theoretical approach that the first-principle calculation to obtain various aspects of drug properties of green tea molecules [60-63].
The first-principle quantum chemical calculations applied to investigation the nature of green tea molecules were performed in the density functional theory (DFT) system using the program package Gaussian 03. At first, the geometry of the molecules was optimized [64-66].
In the all calculations molecule were treated with 6-31G* basis set, carried out by B3LYP function [67,68].
All these cases have been taken into account for several parameters such as energy,dipole moment, quadrupole moment, energy gap, electron energy and etc [69-71].
As a result, energetically favourable structure of four molecules showed on figure 1.
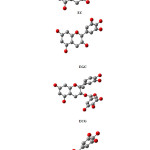 |
Figure 1 Click here to View figure |
NMR calculations also were performed and results were obtained according to output files data.NMR parameters were achieved by following equations:
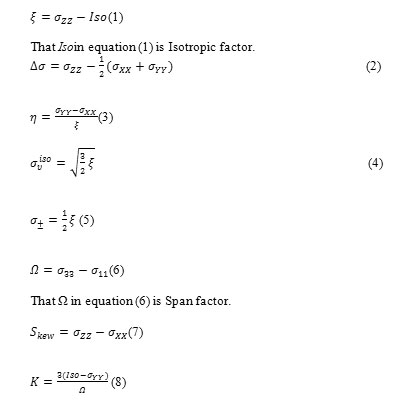
That σYY in equation (8) can be substitute with σ22 for gaining other K factor [72,73].
Results and Discussion
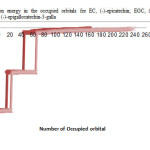 |
Figure2: variation of electron energy in the occupied orbitals for EC, (-)-epicatechin; EGC, (-)-epigallocatechin; ECG, (-)-epicatechin-3-gallate; EGCG, (-)-epigallocatechin-3-gallate. Click here to View figure |
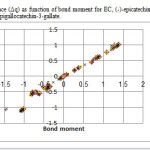 |
Figure3: variation of charge difference (Δq) as function of bond moment for EC, (-)-epicatechin; EGC, (-)-epigallocatechin; ECG, (-)-epicatechin-3-gallate; EGCG, (-)-epigallocatechin-3-gallate. Click here to View figure |
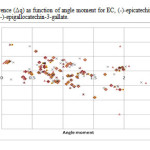 |
Figure4: variation of charge difference (Δq) as function of angle moment for EC, (-)-epicatechin; EGC, (-)-epigallocatechin; ECG, (-)-epicatechin-3-gallate; EGCG, (-)-epigallocatechin-3-gallate. Click here to View figure |
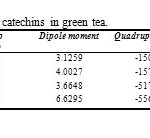 |
Table1: results of optimize calculations for four catechins in green tea. Click here to View table |
The parameters in the NMR were obtained according to output files data. These parameters presented in below tables.
 |
Table2: results of NMR calculations for(-)-epicatechin (EC). Click here to View table |
 |
Table3: NMR parameters for atoms of (-)-epicatechin (EC). Click here to View table |
 |
Table4: results of NMR calculations for (-)-epigallocatechin (EGC). Click here to View table |
 |
Table5: NMR parameters for atoms of (-)-epigallocatechin (EGC). Click here to View table |
 |
Table6: results of NMR calculations for (-)-epicatechin-3-gallate(ECG). Click here to View table |
 |
Table7: NMR parameters for atoms of (-)-epicatechin-3-gallate(ECG). Click here to View table |
 |
Table8: results of NMR calculations for(-)-epigallocatechin-3-gallate (EGCG). Click here to View table |
 |
Table9: NMR parameters for atoms of(-)-epigallocatechin-3-gallate (EGCG). Click here to View table |
References
- M. Monajjemi, and M. Ahmadianarog, Journal of Computational and Theoretical Nanoscience , 11(6), 1465-1471, 2014.
- Reich, Eike; Anne Schibil, Valeria Widmer, Ruth Jorns, Evelyn Wolfram, Alison DeBatt (2006)Journal of Liquid Chromatography & Related Technologies 29 (14): 2141–2151,
- MajidMonajjemi, Robert Wayne, JrandJames E. Boggs, Chemical Physics ,433 (2014)1-11.
- Dattner, Christine; Boussabba, Sophie (2003), Emmanuelle Javelle, ed., The Book of Green Tea, Universe Books, p. 13
- M. MONAJJEMI, M. KHOSRAVI, B. HONARPARVAR , International Journal of Quantum Chemistry, 111, 2771-2777 (2011)
- A. Tahan and M. Monajjemi, ActaBiotheor (2011) 59:291–312
- Wang, L. F.; Zhang, H. Y. BioorgChem 2005, 3, 108.
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J (1999)Am. J. Clin. Nutr. 70 (6): 1040–5,
- Croft, K. D.; Ann, N. Y. AcadSci 1998, 854, 435.
- F. Mollaamin, Z. Varmaghani , and M. Monajjemi, Physics and Chemistry of Liquids. 49(3), 2011, 318–336
- Pietta, P. G. J Nat Prod 2000, 63, 1035.
- M. Monajjemi , N. Karachi & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 22: 643-662, 2014
- Shoeb, M. Bangladesh J Pharmacol 2006, 1, 35.
- Heiss, Mary Lou; Heiss, Robert J. (2007), The Story of Tea: A Cultural History and Drinking Guide, Ten Speed Press, pp. 179–185
- Evans, C. A.; Miller, N. J.; Paganga, G. Free RadicBiol Med 1996, 20, 933.
- Maron DJ, Lu GP, Cai NS, et al. (2003). Arch. Intern. Med. 163 (12): 1448–53.
- M. Monajjem, S. Ketabi, A. Amiri , Russian Journal of physical chemistry , 2006(1),55-62.
- Lambert JD, Sang S, Yang CS (2007), Chem. Res. Toxicol. 20 (4): 583–5,
- Cabrera C, Artacho R, Gimenez R: Benefical effects of green tea: A review. J Am CollNutr 2006, 25:79-99.
- Sano M, Tabata M, Suzuki M, Degawa M, Miyase T, Maeda-Yamamoto M, Analyst 2001, 126:816-820.
- Willson KC: Coffee, Cocoa and Tea. New York: CABI Publishing, 1999.
- Mckay DL, Blumberg JB, J Am CollNutr 21:1-13, 2002.
- Hernandez, E. C.; Naranjo, A. R.; Luis, A.; Cabrera, M. J Mol Struct 2007, 819, 121.
- Fatemeh Mollaamin and Majid Monajjemi, Physics and Chemistry of Liquids ,50(5): 2012, 596–604
- Monajjemi,M. Honarparvar, B. H. Haeri, H. Heshmat ,M. (2006), Russian Journal of Physical Chemistry C.,80(1):S40-S44.
- Nijveldt, R. J.; Nood, E. V.; Hoorn, D. E. C. V.; Boelens, P.G.; Norren, K.; Leeuwen, P. A. M. Am J ClinNutr 2001,74, 418.
- M.Monajjemi,S.Afsharnezhad,M.R.Jaafari,T.Abdolahi,A.Nikosade and Monajemi.Russian Journal of physical chemistry A, 2007(2),1956-1963.
- Manach C, Scallbert A, Morand C, Remesy C, Jimenez L,Am JClinNutr 79:727-747, 2004.
- Ostrowska, J.; Skrzydlewska, E. Adv Med Sci 2006, 51, 298.
- MajidMonajjemi and A.Abedi,Research journal of chemistry and Environment, 2006(4),37-41.
- Wei, T.; Chen, C.; Hou, J.; Zhao, B.; Xin, W. Chin Sci Bull 2000, 45, 422.
- Monajjemi M, Ghiasi R, Abedi A,Russian Journal of Inorganic Chemistry,50(3),382-388 (2005).
- Niemeyer, E. D.; Brodbelt, J. S. J Am Soc Mass Spectrom 2007, 18, 1749.
- M. Monajjemi , A. Sobhanmanesh , & F. Mollaamin,Fullerenes, Nanotubes, and Carbon Nanostructures, 21: 47-63, 2013
- Ogura, R.; Ikeda, N.; Yuki, K.; Morita, O.; Saigo, K.; Blackstock, C.; Nishiyama, N.; Kasamatsu, T. Food ChemToxicol 2008, 46, 2190.

This work is licensed under a Creative Commons Attribution 4.0 International License.









