Antioxidant effect of Purple basil(Lamiaceae) Phenolics
Mohammadi Mastaneh1,*, Majd Ahamd2, Nejadsattari Taher1, Hashemi Mehrdad3
1Department of Plant Science, Faculty of Basic Sciences, Science and Research Campus, Islamic Azad University, Tehran, Iran.
2Department of Biology, Faculty of Biological Sciences, Tehran North Branch, Islamic Azad University, Tehran, Iran.
3Department of Genetics, Tehran Medical Branch, Islamic Azad University, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300459
Article Received on :
Article Accepted on :
Article Published : 20 Nov 2014
Plants used in folk and traditional medicines have been accepted as therapeutic drug development in modern medicine. SinceOcimum basilicum cv. dark opal has been used in Persian traditional medicine and many Iranian dishes,it was considered important to determine the reductive capacity of the purple basil oils and extracts, as this may indicate their potential as antioxidants. Results indicated that the extracts have more powerful antioxidant activity than the oils.Also,the phytochemical analysis of the extracts has led to the identification of 3 phenolic. Our study, partially validates the traditional use of this medicinal herb as complementary medicine.
KEYWORDS:Antioxidant; HPLC; Purple basil
Download this article as:| Copy the following to cite this article: Mastaneh M, Ahamd M, Taher N, Mehrdad H. Antioxidant effect of Purple basil(Lamiaceae) Phenolics. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Mastaneh M, Ahamd M, Taher N, Mehrdad H. Antioxidant effect of Purple basil(Lamiaceae) Phenolics. Available from: http://www.orientjchem.org/?p=5482 |
Introduction
Recently scientists have carried out extensive research into production of herbal drugs using biochemical compounds from herbs. Lamiaceae family including approximately 150 species(1),is well known for antioxidant activity and present antioxidants such as phenolic compounds(2). Among the species which have variation in oil content, composition, and possibly bio activity, Ocimum basilicum(basil) is considered to be promising essential oil crop (3). The basil oil containing pleasant aroma, is known to possess antimicrobial, antioxidant (4, 5)and insecticidal (6)activities and has been used traditionally as a medicinal herb (3).
Basil is also used for pharmaceutical and cosmetic preparations due to the high content of phenolic compounds that are well-known phytochemical molecules found in all plants(7).These constituents act as antioxidants to prevent heart disease(7), reduce inflammation(8, 9), lower the incidence of cancers (10, 11)and diabetes(12,13), as well as reduce rates of mutagenesis in human cells(14, 15). The antioxidant activity of phenolic compounds is mainly caused by their redox properties, which permit them to act as reducing agents, hydrogen donors and singlet oxygen quenchers(16). Rosmarinic acid (RA) is one of the most abundant caffeic acid esters present in Ocimum spp. and have several important biological properties, including antioxidant, antibacterial, antiviral and anti-inflammatory activities(17, 18). P-Coumaric acid (CA) is also reported as an abundant plant phenolic acid and has dietary chemo protectant and antioxidant activity(19). Moreover, it has been shown that ferulic acid (FA) may have potential as a preventative agent against colon tumor development (20).
Purple basil (Ocimum basilicum cv. Dark Opal) is one of the Ocimum basilicumcultivars. However, few studies on antioxidant activity of purple basil essential oils and extracts have been performed until now.Therefore it is important to examine the phenolic acid constituents in purple basil extracts along with the assessment of antioxidant property.
Material and Methods
Plant material
The fresh roots, stems and leaves (prior to flowering),and flowers and the seeds of O. basilicumcv. Dark Opal were collected from a farm at Shahr e Ray city nearby Tehran,in May 2013. After collection, the herb materials were washed with the fresh water and drained under shadow at 18-20 °Cfor 15-25 days and then refrigerated at 4°C.
Extraction
500 mg of dried leaf, stem, and root and flower materials were pulverized separately in a mortar and pestle, suspended in 5 ml of absolute methanol, and left overnight at 4°Cunder dark conditions. All supernatants were decanted and filtered using a What man syringe filter with a 0.45 µm pore size. Samples were rotary-evaporated (45ºC, 10´) to near-dryness and then were stored at -20ºC until used (1).
Essential oils isolation
Essential oils were extracted from Purple basil dried leaf and seed powder samples. 100 g of each dried sample were hydro-distilled in Clevenger-type apparatus (Council of Europe, 1997). Essential oils were distilled for at least 2 h and after collection stored in the dark at -20ºCuntil used.
Phytochemical studies
Rosmarinic acid and other phenolic acids including p-coumaric acid and ferulic acid used as standards were purchased from Sigma-Aldrich Chemicals Co., USA. The HPLC system consisted of a P580 pump (Dionex Co., Sunnyvale, CA), connected to an ASI-100 automated sample injector. A reverse phase C18 column (5 µm particle size, 25 cm × 4.6 mm) was used. The absorbance at 280 nm was measured by a PDA-100 Photodiode array variable UV/vis detector (Dionex Co.).
For HPLC analysis of phenolic compounds, mobile phase solution A consisted of 0.1% TFA (trifluoroacetic acid) in water, and absolute acetonitrile was used as solution B (Fisher Co., USA). A multistep gradient was used for all separations with an initial injection volume of 10 µL and a flow rate of 1 mL min-1. The multistep gradient was as follows: 10% B, 0-5 min; 35% B, 5-20 min; 70% B, 20-40 min; 90% B, 40-41 min; 50% B, 41-42 min; 25% B, 42- 43 min; 5% B, 43-44 min; 100% A, 45 min. Phenolic acids in each sample were identified by comparing retention times to those of authentic standards and were further quantified by comparison of peak area of the standard runs(1).
Antioxidant activity
The reducing power of the oils and extracts was measured according to the method used by Oyaizu with some modification(21). This method is based on the abilities of the present reductants (antioxidants) in each sample to reduce ferricyanide to ferrocyanide and produce a Prussian blue-colored complex (Fe3+) 4(Fe2+ (CN-) 6)3,that is detectable spectroscopically (22, 23, 24).
A volume of 0.5 ml of the extracts and essential oils with different concentrations (1–5 mg/ml) was mixed with 1.25 ml of 0.2 M phosphate buffer (pH 7) and 1.25 ml of 1% potassium ferricyanide. The mixture was incubated at 50°Cfor 20 min followed by the addition of 1.25 ml 10% TCA and then centrifuged for 10 min at 1800 g. Then after, 0.5 ml of the supernatant was mixed with as equal volume of distilled water, followed by adding 0.1 ml of 0.1% ferric chloride (FeCl3). After 10 min the absorbance of the resulting solution was measured at 700 nm. All experiments were performed in triplicate.
Statistical analysis
All data were expressed as mean±SE for three experiments. The difference between the mean±SE of the antioxidant activity between the control (without plant extract) and experimental group were assessed using the one way ANOVA and Tukey Post Hoc test. P values < 0.05 were considered statistically significant. Statistical analyses were performed using Excel software and SPSS version 18.0 for Windows 2007.
Result and Discussion
As can be seen from Figure 2, although all the three extracts and two oils showed some degree of reduced Fe3+ to Fe2+, the extracts were the more effective at reducing their on (III), with an absorbance reading in range of 0.273 ±0.008 to 1.344 ±0.055 rather than the oils. The activity for the oils was in range of 0.05±0.010 to 0.410±0.010 (P < 0.05).It seems the methanolic extracts have a considerable ability to react with free radicals to alter them into more stable non-reactive species.
According to the phytochemical analysis results, rosmarinic acid was identified and quantified in all three the extracts of purple basil, and also was the major component identified in comparison with other compounds.This is in general agreement with previous studies which reported that rosmarinic acid is the most abundant component in O. basilicum(1).
We detected significant levels of rosmarinic acid in methanolic extract of roots (97.59±.0141 µg/ml), that was the highest concentration of RA observed of the parts of purple basil tested. This may explain the high antioxidant activity of the root extracts (Table 1). This result is similar with Kiferle study(25)in which the level of RA in root extracts was higher than the leaves extracts in Ocimum basilicum, and also is similar with Jayasing he (26)study in which the major antioxidant compound was confirmed asRA. However, the flower extract was also rich in RA and the level was close to that of root extract which explains its powerful antioxidant effect (Figures 1B and 2).
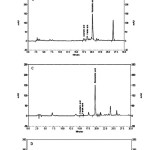 |
Figure 1 Click here to View figure |
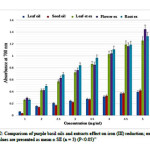 |
Figure 2Click here to View figure |
P-coumaric acid and ferulic acid were also identified but were quantified only in the flower extracts, although they were still only present in low quantities compared to rosmarinic acid level (Table 1) (Figures 1 A-D).This result is similar with Javanmardi (1)study where these phenolic compounds levels were higher in flower extracts than that of leaves samples. Also it is similar with Benedec(27)in which CA and FA were found in small quantities in leaf and stem samples of Ocimum basilicum, which explains the antioxidant ability of flower extract.
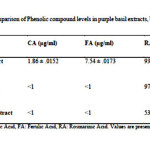 |
Table 1Click here to View table |
Although the antioxidant effect of flower extract was close to that of root sample, the antioxidant activity of flower sample was increased, by using higher concentration (5 mg/ml) of the extract. It seems that the presence of more quantity of CA and FA in flower sample must be the reason(Figures 1B and 2).
Leaf extract of purple basil showed a lower amount of RA whilst also having a powerful antioxidant activity. This is in general agreement with Dorman (28)who explained Ocimum basilicum leaf extracts have capable antioxidant effect.
Our results revealed that methanolic extracts of purple basil have antioxidant activity which is concentration-dependent, and that phenolic compound are responsible for this effect. This is similar with previous studies, where different species of Ocimum have been investigated (16, 29).
Purple basil essential oils at various concentrations were also determined. As can be seen in Figure 2, both two purple basil oils possess considerable reducing power and through the increasing of the concentrations, absorbance level was growing up. This result is similar with Trevisan et al study(30). Statistical analysis indicated that the antioxidant activity of the leaf and seed oils were similar. However the antioxidant activity of extracts with the mean absorbance level of 0.733± 0.081 was 3.14 fold more than that of oils (Figure 2). Juliani and Simon (31)evaluated the antioxidant activity of different basil essential oils and in all basils the essential oil contribution to the total antioxidant activity was low.
In conclusion, all extracts and oils were found to exhibit a good antioxidant activity in the selected invitro antioxidant assay. Overall, the oils tend to possess lesser activity in comparison to the extracts. However essential oils and their main components possess a wide spectrum of biological activity(32).
These observations prompt the necessity for further research studies, focusing on the isolation and structural elucidation of their pure antioxidant compounds.
References
- Javanmardi, J.;Khalighi, A.;Kashi, A.;Bais, H. P.;Vivanco, J. M. J. Agric. Food Chem. 2002,50, 5878-5883
- Skrovánková, S.;Mišurcová, L.;Machů, L. Adv Food Nutr Res.2012, 67, 75-139
- Simon J. E.; Morales M. R.;Phippen W. B.; Vieira R. F. andHao Z., Perspectives on New Crops and New Uses, ASHS Press, Alexandria. VA,(1999)
- Bozin, B.;Mimica-Dukic, N.;Simin, N.;Anackov, G. J. Agric. Food Chem.2006,54, 1822-1828
- Suppakul, P.;Miltz, J.;Sonneveld, K.; Bigger, S. W. J. Agric. Food Chem. 2003,51, 3197-3207
- Aslan, I.;Ozbek, H.;Calmasur, O.;Sahin, F. Ind. Crop. Prod.2004,19, 167–173
- Simon J. E.; Chadwick A. F. andCraker L. E., The scientific literature on selected herbs, and aromatic and medicinal plants of the temperate zone, Archon Books. Hamden,(1984)
- Mohanlal, S.;Parvathy, R.;Shalini, V.;Mohanan, R.; Helen, A.;Jayalekshmy, A. J. Food Biochem. 2012, 36, 1–12
- Jin, X. H.;Ohgami, K.;Shiratori, K.; Suzuki, Y.; Koyama, Y.; Yoshida, K.; et al. Exp. Eye Res.2006,82, 860–867
- Sawadogo, W. R.;Maciuk, A.;Banzouzi, J. T.;Champy, P.;Figadere, B.;Guissou, I. P.; et al. Nat. Prod. Res.2012,26, 575–579.
- Slivova, V.;Zaloga, G.;DeMichele, S. J.;Mukerji, P.; Huang, Y. S.; Siddiqui, R.; et al. Nutr. Cancer. 2005,52, 66–73
- Kusirisin, W.;Srichairatanakool, S.;Lerttrakarnnon, P.;Lailerd, N.; Suttajit, M.;Jaikang, C.; et al. Med. Chem. 2009,5, 139 147
- Scalbert, A.;Manach, C.;Remesy, C.;Morand, C. Crit. Rev. Food Sci. Nutr.2005,45, 287–306
- Pedreschi, R.; Cisneros-Zevallos, L. J. Agric. Food Chem.2006,54, 4557–4567
- Gomez-Cordoves, C.; Bartolome, B.; Vieira, W.; Virador, V. M. J. Agric. Food Chem. 2001,49, 1620–1624
- Hakkim, F. L.; Shankar, C. G.;Girija, S. J.Agric.food chem,2007,55, 9109
- Pereira, D. M.;Valentão, P.; Pereira JA, Andrade PB. Molecules.2009, 2202–2211
- Juliani H. R.;Koroch A. R. and Simon J. E. Dietary Supplements, American Chemical Society Symposium Series 987, American Chemical Society. Washington D.C.,(2008)
- Torres, Y.; Torres, J. L.;Rosazza, J. P. J. Agric. Food Chem.2001, 49, 1486-1492
- Han, B. S.; Park, C.B.;Takasuka, N.; Naito, A.;Sekine, K.; Nomura, E.; et al. Jpn. J. Cancer Res.2001, 92, 404-409
- Oyaizu, M. Jpn. J.Nut. 1986,44, 307–315
- Kumar, S.; Kumar, D.; Prakash, O. J. Environ. Agric. Food Chem.2008,7, 2863-71
- Hinneburg, I.; Dorman, D. H. J.;Hiltunen, R. Food Chem.2006,97, 122-129
- Chung, Y. C.; Chang, C. T.; Chao, W. W.; Lin, C. F.; Chou, S. T. J. Agric. Food. Chem. 2002,50, 2454–2458
- Kiferle1, C.; Lucchesini1, M.;Mensuali-Sodi, M.; Maggini1, R.;Raffaelli, A.; Pardossi1, A. Cent. Eur. J. Biol. 2011, 6, 946-957
- Jayasinghe, C.;Gotoh, N.; Aoki, T.; Wada, S. J. Agric. Food. Chem.2003, 51, 4442-4449
- Benedec, D.;Vlase, L.;Hanganu, D.;Oniga, I. Digest Journal of Nanomaterials and Biostructures.2012,7, 1263 – 1270
- Dorman, H. J.;Hiltunen, R. Nat Prod Commun. 2010,5, 65-72
- Arivazhagan, G.; Hakkim, F. L.;Boopathy, R. J. Med. Plants Res.2008.2, 250-257
- Trevisan, M. T. S.; Silva, M. G. V.;Pfundstein, B.; Spiegelhalder, B.; Owen, R. W. J. Agric. Food Chem. 2006,54, 4378-4382
- Juliani H. R. and Simon J. E., Trends in new crops and new uses, ASHS Press, Alexandria. VA,(2002)
- Cristani, M.;d’Arrigo, M.; Mandalari, G.;Castelli, F.;Sarpietro, M. G.;Micieli, D. J. Agric. Food Chem.2007, 55, 6300-6308

This work is licensed under a Creative Commons Attribution 4.0 International License.









