Effect of Dimethoxy-Methane (C3H8O2) on Combustion Characteristics of a Direct Injection Diesel Engine with Variable Compression Ratio Fuelled with Biodiesel Blends with Diesel (C10.8H18.7)
M. Prabhahar1, S. Sendilvelan2 and J. Francis Xavier3
and J. Francis Xavier3
1Department of Mechanical Engineering, Aarupadai Veedu Institute of Technology, Chennai-603104, (Tamilnadu) India.
2Department of Mechanical Engineering, Dr. M.G.R. Educational and Research Institute, University, Chennai- 600095, (Tamilnadu) India.
3Department of Mechanical Engineering, Veltech Dr.R.R. and Dr.S.R. University, Chennai-600062, (Tamilnadu) India.
Corresponding Author E-mail: sendilvelan.mech@drmgrdu.ac.in
DOI : http://dx.doi.org/10.13005/ojc/330629
It is necessary to find a fuel source due to the heavy usage of natural resources. Developing countries have been trying various sources and methods to identify suitable alternative fuel for engines. This paper investigates the utilization of Palm Oil Methyl Ester (POME) blends with diesel in Variable Compression Ratio (VCR) diesel engine. The performance and emission characteristics of biodiesel with 10% dimethoxy methane additive (20% Palm Oil Methyl Ester biodiesel with 10% DMM) for various compression ratios (15.5,16.5 and 17.5) have been studied and it was found out that the blends could substitute for diesel and used as an alternate source for the future generation. Emission parameter like CO, HC, NOx and Soot was evaluated and conclusions were drawn.
KEYWORDS:Palm Oil Methyl Ester; Dimethoxy Methane; Variable Compression Ratio; Diesel Engine; Emission Reduction
Download this article as:| Copy the following to cite this article: Prabhahar M, Sendilvelan S, Xavier J. F. Effect of Dimethoxy-Methane (C3H8O2) on Combustion Characteristics of a Direct Injection Diesel Engine with Variable Compression Ratio Fuelled with Biodiesel Blends with Diesel (C10.8H18.7). Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Prabhahar M, Sendilvelan S, Xavier J. F. Effect of Dimethoxy-Methane (C3H8O2) on Combustion Characteristics of a Direct Injection Diesel Engine with Variable Compression Ratio Fuelled with Biodiesel Blends with Diesel (C10.8H18.7). Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40185 |
Introduction
There are some issues due to technology advancement relating to an alternate fuel to sustain the automobile sector for the future. However, our dependence is on diesel and petroleum for fueling the transportation sector and if this continues then this could threaten our energy resource, affect our economy and even affect our environment so badly that it may even take hundreds of years for a seed to sprout. Thus, in search of an alternate source of fuel to have a sustainable economy1. This is possible with the use of biodiesel which is a renewable source of energy. Though it is not possible to run a CI Engine on 100% biodiesel like Jatropha and Pongamia without any major modifications in the presently available engine, when blended with diesel in various proportions it would make the world wonder with its Eco-friendly nature2. Biodiesel is nothing but long-chain alkyl esters which is obtained from animal fat and plant seeds. They are regarded as carbon sink as they absorb 78.5% of carbon in the atmosphere as they burn and even considered as cleaner than fossil fuels3.
Waste oil biodiesel has showed an increase in fuel density and decrease in calorific value of fuel and improved power, thermal efficiency and reduction in the specific fuel consumption4. Waste cooking oil could be produced through transesterification process5. Pugazhvadivu and Jeyachandran concluded that heating the frying oil to about 135 °C reduces the viscosity to that of diesel at 30°C and it could be used as an alternative fuel in diesel engine6. Horng-Wen Wu et al. used Taguchi method on combustion performance of a diesel engine with biodiesel blends7. Preheated Palm Oil Methyl Esters (POME) in the diesel engine shows improved brake power output and engine performance8. Ignition delay, pressure rise and peak pressure were close to diesel combustion at the constant engine load and speed. The specific fuel consumption values of the methyl esters were less than that of the raw vegetable oils and high specific fuel consumption in vegetable oils is due to lower energy content9. Biodiesel is a mixture of glyceride free long chain fatty acids obtained from oils and fats study report that there are some inconsistencies in the NOx emission behavior among petroleum diesel and biodiesel10.
Rakopoulos et al. concluded that some minor effect on the transient performance of the turbo charged diesel engine and also in the combustion noise radiation when biofuels blends are used11. The biobutanol from food wastes as biofuel showed that there is an increase in CO, hydrocarbons running the engine on lower loads emit lesser NOx contents. Zhu et al. investigated diesel dimethoxy methane (DMM) blends experimentally and reported that the effect of fuel constituents on combustion characteristics, fuel efficiency and emissions of a diesel engine12. He also reported that with the use of DMM, CO and smoke emissions can be reduced significantly, while HC emissions and particulate matter increase slightly. Roy et al. investigated to improve the performance of a diesel engine by adding oxygenated fuel additive to control the emission and to improve its performance13. Previous research showed that the reduction of PM and NOx could be achieved by using DMM blends. However, these studies were performed only for diesel and DMM blends, and only few researches have done experimental work on Palm Oil Methyl Ester and DMM blends. So, in this work the utilization of Palm Oil Methyl Ester (POME) and DMM with diesel in Variable Compression Ratio (VCR) diesel engine have been analyzed. The performance and Combustion characteristics of B20PAMDMM (20% Palm Oil Methyl Ester and 75% Diesel 5% DMM) for various compression ratios (15.5,16.5 and 17.5) have been studied.
Oxygenated Additives
Fuels that have a chemical compound that contains oxygen atoms are called oxygenated additives. Effective burning is possible with the use of oxygenated additives and also the major benefit is it cut down the emission level comes out from the engine exhaust. At high engine loads, oxygenated additive shown to be more effective than low engine loads. The combustion efficiency increases with higher oxygen concentration in the fuel, which in turn indicate reduction of HC, CO and other exhaust pollutants.
Miyamoto et al. measured emissions concentrations with four oxygenates as additives to diesel fuel and identified that zero smoke emission at 30% of weight oxygenate addition with 0.75 MPa mean effective pressure14. Also reported that particulate reduction not related to fuel type but it was related to concentration of oxygen.
Oxygenated additive also helps to reduce the utility of non-renewable fossil fuels, which in turn reduces the environmental pollution. In recent years, dimethoxymethane (DMM) has been a promising alternative diesel fuel, since it has a high oxygen fraction and high cetane number. Such positive advantages make DMM a good oxygenated additive to be used in diesel engines.
In this study, dimethoxy methane (CH3OCH2OCH3, DMM) were analyzed and reported. DMM being non-toxic and miscible with diesel fuels. Dimethoxymethane (DMM) is a high oxygen content additive and also has high cetane number, which makes DMM a good oxygenate additive for diesel/oxygenate fuel blends.
Experimental
The research engine test setup with following configuration has been used for this work. Single cylinder, four stroke, Multifuel water cooled VCR engine. Stroke:110mm, Bore:87.5mm, Capacity 661cc, Power 3.5 kW, speed 1500 rpm, CR range 12:1-18:1. Eddy current dynamometer for loading. Experiments were conducted at 17.5, 16.5 and 15.5 compression ratios with the rated power output of the engine. Before each measurement, the engine was warmed up and running steadily. The brake thermal efficiency, NOx, HC, CO emissions as well as the opacity in percentage were recoded and analyzed in this study. Figure 1 shows the schematic experimental system and measuring instruments used in this work.
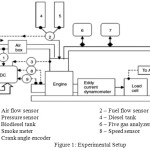 |
Figure 1: Experimental Setup |
Results and Discussion
Figure 2 shows the variation of fuel consumption in kg/hr with varying compression ratio 15.5,16.5 and 17.5 at the rated power output. It can be observed that the fuel consumption is an indication of the efficiency with which the power developed by the engine defined. It is found that compression ratio of 16.5 gives better fuel consumption than the other two. The specific fuel consumption with diesel is 0.32 kg/hr compared with the 20% PAM Biodiesel and10% DMM as 0.37kg/hr at 16.5 compression ratio, which is lower than any other mode other than Diesel.
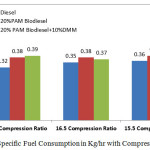 |
Figure 2: Specific Fuel Consumption in Kg/hr with Compression Ratio Click here to View figure |
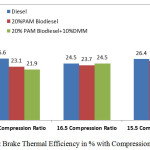 |
Figure 3: Brake Thermal Efficiency in % with Compression Ratio |
Figure 3 shows that the variation of brake thermal efficiency with three different compression ratios at rated power output. The brake thermal efficiency is higher at 16.5 compression ratio with 20% PAM with 10% DMM compared to the 17.5 compression ratio. The results, revealed that with 20%PAM biodiesel 10%DMM shows lower brake thermal efficiency compared with the other two modes. In the case of 16.5 compression ratio 20%PAM biodiesel 10%DMM blends shows comparable value of brake thermal efficiency with the diesel fuel. 20% PAM shows better efficiency at 15.5 compression ratio as 25.5 % compared with the diesel. By adding dimethoxy methane with palm oil is not effective in the cases of 17.5 and 15.5 compression ratios. The brake thermal efficiency increases in the case of 16.5 compression ratio, but still it is lower than diesel. This may be due to the PAM addition which has lower calorific value than the diesel.
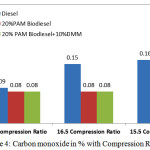 |
Figure 4: Carbon monoxide in % with Compression Ratio Click here to View figure |
Figure 4 shows the variation of carbon monoxide with three different compression ratios at rated power output. The higher compression ratio increases better combustion which reduced the carbon monoxide in % by volume. The exhaust CO concentration in percentage for 20% PAM, 20% PAM with 10% DMM fuel blends at 17.5 compression ratio and 16.5 compression ratio are same as 0.08%. The value increases with reduced compression ratio of 15.5. This may be due to oxygen availability of PAM oil methyl ester. Carbon monoxide (CO) gas is toxic products produced from all hydrocarbon combustion which can be reduced by higher oxygenated nature of fuel include biodiesel. Complete oxidation of the fuel produces a more complete combustion and contributes to lower emission of carbon monoxide (CO).
Figure 5 gives HC emissions in ppm with varying compression ratio 15.5,16.5 and 17.5 at the rated power output. The HC concentration is low in the case of 20% PAM blends obviously PAM oxygenated fuel supports better combustion with diesel.
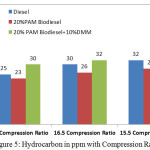 |
Figure 5: Hydrocarbon in ppm with Compression Ratio |
The expected reduction of HC not happened with addition of 10% DMM considered as higher oxygen mass (8.46). This may be due to the boiling point of DMM, which is 40°C below the diesel. DMM disperses all areas before the flame reaches, which could be the reason for higher HC. For diesel, the HC emissions vary from 25 ppm 32 ppm. For 20% PAM, 20% PAM with 10% DMM the variations are from 23 ppm to 28 ppm, 30 ppm to 32 ppm respectively.
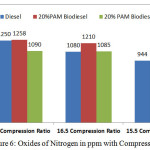 |
Figure 6: Oxides of Nitrogen in ppm with Compression Ratio Click here to View figure |
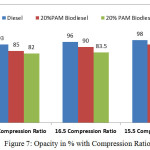 |
Figure 7: Opacity in % with Compression Ratio |
Figure 6 and 7 shows the variation of oxides of nitrogen in ppm and opacity in % with varying compression ratio 15.5,16.5 and 17.5 at the rated power output. The oxides of nitrogen in ppm and the opacity in % reduces with 16.5 compression ratio. But the compression ratio reduces the value of oxides of nitrogen and opacity increases considerably. The cetane number for palm oil methyl ester is 52 compared with 45 cetane number for the diesel fuel. Experiments with biodiesel found that a higher proportion of biodiesel attributes to increasing in NOx emission due to higher oxygenated nature content in biodiesel fuel. But by adding 10%DMM with the blends slightly reduced the NOx emissions.
The chemical nature of the fuel which contains high level of oxygen and the absence of the aromatic molecules in the fuel composition has reduced the formation of the soot particles with the increasing in oxidation rate, which in turn reduce the opacity percentage. With compression ratio of 16.5 the opacity is marginally reduced as 83.5 in percentage compared with 96% in the case of diesel fuel and 91% in the case of 20%PAM biodiesel blends as shown in Figure 7.
Conclusion
The aim of the investigation was successfully carried out and the following conclusions were drawn: Biodiesel produced from Palm Oil Methyl Ester (POME) can be successfully used as alternative fuel in the existing diesel engines without any major modifications. The addition of DMM reduces the emission of oxides of nitrogen and opacity. Better fuel economy was observed at 16.5 compression ratio compared to other compression ratios. It is concluded that the blends of 20% PAM with 10% DMM as an alternative fuel in a variable compression ratio engine shows the optimum performance and emission characteristics with 16.5 compression ratio.
References
- Sassykova, L.; Gil’Mundinov, Sh.; Nalibayeva, A.; Bogdanova, I. Rev. Roum. Chim. 2017, 62 (2), 107–114.
- Sassykova, L.R.; Ussenov, A.; Massenova, A.T.; Gil’Mundinov, Sh. A.; Rakshmetova, K.S.; Bunin, V.N.; Basheva, Zh.T.; Kalykberdiyev, M.K. Int. J. Chem Sci. 2016, 14 (1), 206–212.
- Baiseitov, D. A.; Tulepov, M. I.; Sassykova, L. R.; Gabdrashova, Sh.E.; Gul’Dana, E.; Zhumabai, D.A.; Kudaibergenov, K. K.; Mansurov, Z. A. Int. J. Chem. Sci. 2015, 13 (2), 1027–1033.
- Yamin, J. A.; Sakhnini, N.; Sakhrieh, A.; Hamdan, M. A. Sustain. Cities and Soc. 2013, 9, 32–38.
CrossRef - El-Kasaby, M.; Nemit-Allah, M. A. Alexandria Eng. J. 2013, 52 (2), 141–149.
CrossRef - Pugazhvadivu, M.; Jeyachandran, K. Renew. Energy 2005, 30 (14), 2189–2202.
CrossRef - Wu, H. W.; Wang, R. H.; Ou, D. J.; Chen, Y. C.; Chen, T. yu. Appl. Energy 2011, 88 (11), 3882–3890.
CrossRef - Agarwal, A. K. Prog. Energy Combust. Sci. 2007, pp 233–271.
- Sendilvelan, S.; Bhaskar, K. Rasayan J. Chem. 2016, 9 (4), 692–696.
- Sendilvelan, S.; Bhaskar, K. Rasayan J. Chem. 2017, 10 (1), 111–116.
- Rakopoulos, D. C.; Rakopoulos, C. D.; Giakoumis, E. G.; Dimaratos, A. M.; Kyritsis, D. C. Energy Convers. Manag. 2010, 51 (10), 1989–1997.
CrossRef - Zhu, L.; Cheung, C. S.; Zhang, W. G.; Huang, Z. Fuel 2011, 90 (5), 1743–1750.
CrossRef - Roy, M. M.; Wang, W.; Alawi, M. Energy Convers. Manag. 2014, 84, 164–173.
CrossRef - Miyamoto, N.; Ogawa, H.; Nabi, M. Int. J. Engine Res. 2000, 1 (1), 71–85.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









