Separation and Determination Micro Amount Cd+2 Ion by Using Cryptand C221 Via Liquid Ion Exchange Methodology
Shawket Kadhim Jawad and Maha Abbas Hussien
Department of Chemistry, College of Education for Women, Kufa University, Al-Najaf- 31001, Iraq.
Corresponding Author E-mail: Shawkat.alshakarchi@uokufa.edu.iq
DOI : http://dx.doi.org/10.13005/ojc/330538
By using cryptand C221 as organic reagent for separation and extraction Cd+2 ion was performed by liquid ion exchange method, spectrophotometric study for ion pair association complex extracted to organic phase was elucidated wave length for maximum absorbance was λmax=372nm, as well as experiments to pinpoint optimum condition shows 0.25M KOH with existence 100µg Cd+2 0.25M 8-HQ at shaking time 15 min. with 1×10-4M C221 dissolved in chloroform the research demonstrate by presenting method in aqueous solution effect to increase extraction efficiency, so that the extraction method was endothermic method, so the experiments shows there in un effect for organic solvent and the more probable structure of ion pair complex extracted was 1:1:1 potassium ion:C221:Cadmium oxin anion complex [K:C221]+; Cd(OX)-2.
KEYWORDS:Cryptand C221; Cadmium (II); Liquid ion Exchange; Separation; Preconcentration
Download this article as:| Copy the following to cite this article: Jawad S. K, Hussien M. A. Separation and Determination Micro Amount Cd+2 Ion by Using Cryptand C221 Via Liquid Ion Exchange Methodology. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Jawad S. K, Hussien M. A. Separation and Determination Micro Amount Cd+2 Ion by Using Cryptand C221 Via Liquid Ion Exchange Methodology. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38754 |
Introduction
Solvent extraction of Cd (II) from sulfate medium by using bis (2-ethylhexyl) phosphoric acid (D2EHPA) dissolved in toluene. Cadmium is extracted by a cation-exchange mechanism as CdR2.2HR1. Cadmium picrate CdPic2 in water was extracted by 18-crown-6 into o-dichlorobenzene, bromobenzene, dibutylether, and nitrobenzene. This study, an extraction constant for an HPic extraction was determined spectrophotometrically at 25°C 2.
Extraction of Cd2+ and Hg2+ as chloro anion complexes CdCl4=, HgCl4= and HgCl3– from hydrochloric acid medium by using many extractants according to liquid ion exchange method was α-Naphthyl amine, 4-Amino benzoic acid, 2-[(4-Carboxy methyl phenyl) azo]-4,5-diphenyl imidazole and Cryptand (C222). Stoichiometry showed the ion pair complex extracted was 1:1:1 Cation: Ligand: Anion 3. Determination of Mg (II) in different environmental and vital samples by using 1-(2- Pyridyl azo)-4-Benzene naphthol(PABN), after conversion Mg2+ to anion complex by reaction with oxine, initially the organic reagent(PABN) must be react with Ni (II) to form large cation complex produce ion pair complex with Mg(OX)3– 4. Rhodamine 6G used as anion exchanger for extraction of Hg2+ and Zn2+ as chloro anion complexes from HCl media. This method applied for determination this metal in different samples5. Extraction of Cd (II) from aqueous solutions by using organic reagent 4-carboxy-methyl phenyl) azo]-4,5-diphenyl imidazole at pH = 10, with shaking time 5 min. this study showed the ion pair complex extracted for Cd2+ has sandwich structure 1:2 metal:ligand [Cd(4-CMePAI)2]2+; anion6. The extraction of Cd(II) by using 2-[Benzothiazolylazo]-4-benzylphenol, the result shows optimum pH was 9, and 50μg Cd+2 ion, shaking time was 10min., as well as stoichiometry studies show the more probable structure of complex extracted was 1:1 Metal:Ligand [Cd+2(BTABP)(Cl–)], in addition to synergism effect shows participate one molecule of TBP in each molecule of complex formed and effect to enhanced distribution ratio (D) [Cd+2(BTABP)(Cl–)(TBP)] 7.
Experimenital
Material and Methods
A biochrom double beam UV-visible spectrophotometer model biochrom libra 560 Cambridge UK with 1 cm path length used for all spectrophotometric studies as well as all absorbance measurements, Electrical balance CE, HR 200 (Japan) used for all weight, Electrical shaker HY – 4- Vibrator, with AD Just about speed multiple Japan.
Used distilled water for prepared all solutions with suitable volumetric flasks stock solution of Cd+2 ion at concentration 1000µg/ml prepared by dissolved 0.1631 g of CdCl2.H2O in 100 mL distilled water contain 1 mL of cons. HCl by used volumetric flask 100 mL in volume other working solution prepared by dilution with distilled water in sufficiently volumetric flasks. 2M 8-Hydroxy quinoline prepared by dissolved fixed quantity in chloroform by used volumetric flask. Cryptand C221 preparing at 1×10-2 M by dissolved adequate weight in suitable volume of chloroform by used volumetric flask. And other solution for extraction and determination prepared as needed.
General Method
Aqueous solution 5mL in volume contain 100µg of Cd+2 ion and 0.25M of KOH and shaking with 5ml of chloroform organic solvent contain 1×10-4M of cryptand C221with 0.25M 8-Hydroxy quinolone for 15 min. in electrostatic shaker, then separated the organic phase from the aqueous phase and measure the absorbance of organic phase at λmax of ion pair association complex extracted against blank prepared at the same manner in absence metal ion. But the aqueous phase treated according to dithizone spectrophotometric method 8. And after the return to the calibration curve Figure 1 determine the remainder quantity of Cd+2 ion in the aqueous phase after extraction then subtraction this quantity from the origin quantity of the metal ion Cd+2 to determine the transferred quantity of metal ion Cd+2 into organic phase to formation ion pair association complex afterward calculate the distribution ratio D for extraction as thermodynamic criterion for extraction efficiency of Cd+2 ion any application the relation below:
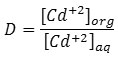
Results and Discussion
Spectrophotometric study for ion pair association complex extracted to the organic phase shows the wave length for maximum absorbance was λmax=372 nm as in the spectrum in Figure 2.
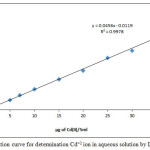 |
Figure 1: Calibration curve for determination Cd+2 ion in aqueous solution by Dithizone method |
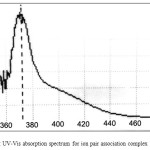 |
Figure 2: UV-Vis absorption spectrum for ion pair association complex extracted |
Effect of KOH Concentration
100µg Cd+2 ion in 5 mL aqueous solution contain rising concentration of base KOH, by shaking with 5mL chloroform solution contain 1×10-4 M C221 and 0.25M 8-Hydroxy quinoline for 15min. and then complete the procedure according to the general method the results were as in Figure 3,4:
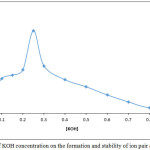 |
Figure 3: Effect of KOH concentration on the formation and stability of ion pair association complex |
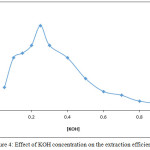 |
Figure 4: Effect of KOH concentration on the extraction efficiency |
The results show 0.25 M KOH was the optimum concentration of base in aqueous solution giving higher extraction efficiency this concentration was more suitable for equilibrium formation anion complex of Cd+2 as Cd(OX)3– at higher concentration and formation higher concentration of ion pair complex with cryptand C221 according to thermodynamic relations below:
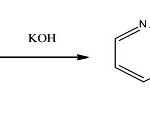 |
Scheme 1 |
Cd+2 + 3OX– ↔ Cd(OX)3–
C221 + KOH ↔ [K-C221] +; OH–
[K-C221]+; OH– + Cd (OX)3– ↔ [K-C221]+;Cd(OX)3– +OH–
Any concentration of KOH less than optimum not suitable to reached favorable equilibrium and effect to decrease extraction efficiency, so that any concentration of KOH more than optimum value effect to decline extraction efficiency also because this concentration effect to prevent formation of ion pair complex by masking K+ ion and not bonding with C2219.
Effect Metal Ion Concentration
Extraction Cd+2 ion from 5 mL aqueous solution contain rising quantity of this metal ion and 0.25 M KOH after shaking with 5mL chloroform solution contain 1×10-4 M C221 and 0.25M 8-HQ as detailed in general method the results were as in Figures 5,6:
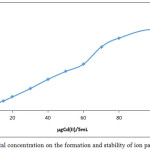 |
Figure 5: Effect of metal concentration on the formation and stability of ion pair association complex |
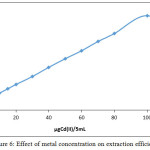 |
Figure 6: Effect of metal concentration on extraction efficiency |
The results show there is arising in ion pair association complex and extraction efficiency with increasing metal ion concentration until 100µg Cd+2/5mL which in the optimum concentration of metal ion allow to reached favorable equilibrium for extraction and any concentration more than this optimum value according to mass action law effect to decrease extraction efficiency9.
Effect of 8-HQ Concentration
According to general method extracted 100μg Cd2+ in 5mL aqueous solutions contain 0.25M KOH by shaking in electrostatic shaker for 15min. with 5mL chloroform contain 1×10-4M C221 and rising concentrations of 8-HQ. The results were as in Figures 7, 8:
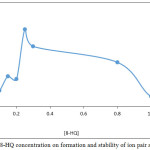 |
Figure 7: Effect of 8-HQ concentration on formation and stability of ion pair association complex |
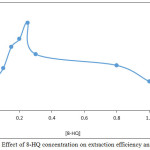 |
Figure 8: Effect of 8-HQ concentration on extraction efficiency and D values |
The results appear 0.25M 8-HQ was the optimum concentration effect to give favorable and best equilibrium for formation ion pair association complex any concentration less than optimum giving decrease in extraction efficiency so that any concentration more than optimum giving decline in extraction efficiency also because this concentrations effect to decrease anion complex Cd (OX)3– and decrease ion pair complex formation and stability9.
Shaking Time Effect
From 5mL aqueous solution 100μg Cd2+ was extracted at all optimum condition according to general method except at different shaking times the results were as in Figures 9,10:
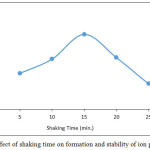 |
Figure 9: Effect of shaking time on formation and stability of ion pair complex |
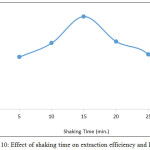 |
Figure 10: Effect of shaking time on extraction efficiency and D values |
The results show 15min. was the optimum shaking time to give maximum extraction efficiency with high concentration and stability of ion pair complex extracted, any shaking time as kinetic side of extraction method less than optimum not allow to reach best thermodynamic equilibrium, so that shaking time more than optimum effect to decrease the extraction efficiency by effect of increase backward direction of equilibrium and increase dissociation of complex.
Effect of Cryptand C221 Concentration
Extracted 100μg Cd2+ ion from 5mL aqueous solutions at optimum conditions by using rising concentrations of Cryptand C221 according to general method. The results demonstrated in Figures 11,12:
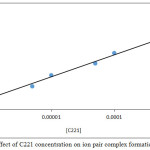 |
Figure 11: Effect of C221 concentration on ion pair complex formation and stability |
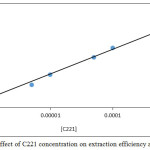 |
Figure 12: Effect of C221 concentration on extraction efficiency and D values |
The results show there is a linear relation effect for rising concentration of C221 on formation and stability of ion pair complex extracted so that extraction efficiency, whereas C221 concentration is thermodynamic parameter effect with increasing concentration to increase the rate of forward direction for thermodynamic equilibrium to formation ion pair association complex.
Effect of Methanol
100μg Cd2+ in 5mL aqueous solutions was extracted according to general method in the presence rising percentage of methanol CH3OH in aqueous solutions. The results were as in Figures 13, 14:
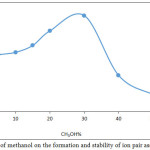 |
Figure 13: Effect of methanol on the formation and stability of ion pair association complex |
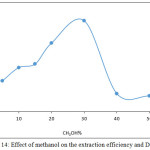 |
Figure 14: Effect of methanol on the extraction efficiency and D values |
The results show there is an increase in extraction efficiency in the presence of methanol in aqueous solution and the maximum increasing reached at 30% CH3OH and decreasing after that because methanol in aqueous phase to give rise to decrease the polarity and dielectric constant of aqueous solution and destroyed the hydration shell of metal ion this effect to decrease the forward direction of equilibrium and increase the formation of ion pair association complex and reached maximum behavior at 30% CH3OH any percentage more than optimum value effect to decrease extraction efficiency because the very more decline in polarity of aqueous solution effect to transfer some of cryptand C221 to the aqueous phase and decrease it concentration in organic phase so that increase the ion pair association complex in organic phase and extraction efficiency.
Effect of Temperature
100μg Cd2+ ion in 5mL aqueous solutions was extracted according to general procedure at different temperature. The results were as in Figures 15,16:
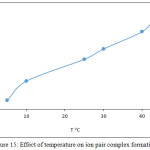 |
Figure 15: Effect of temperature on ion pair complex formation |
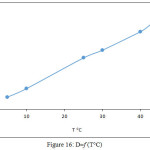 |
Figure 16: D=f (T°C) |
After calculated extraction constant according to the relation below, the results demonstrate in Figure 17:
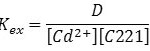
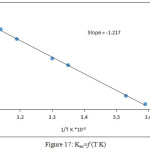 |
Figure 17a: Kex=f (T K) Click here to View figure |
From the slope of straight line relation of Kex with 1/T K and the relations below calculated thermodynamic data10 as in Table 1:
![]()
∆Gex = – R T ln Kex
∆Gex = ∆Hex – T∆Sex
Table 1: Thermodynamic data for extraction Cd2+ ion
| Δ Hex kJ/mol | Δ Gex kJ/mol | ΔSex J/mol/K |
| 0.023 | -58.38 | 183.66 |
The results appear extraction method was endothermic and the small value of enthalpy ΔHex appear there is an effective approach between ions in ion pair association complex with high stability, so that high value of entropy show the method was entropic in region.
Effect of Organic Solvent
100μg Cd2+ ion in 5mL aqueous solutions was extracted according to general method by using different organic solvents for cryptand C221 differ in dielectric constant. The results were as in Table 2:
Table 2: Effect of organic solvents on extraction efficiency of Cd2+ ion
|
Organic solvent |
εr |
Wave length nm |
Abs |
D |
|
Amyl alcohol |
15.8 |
442 |
0.270 |
4.38 |
|
1,2-Dichloro methane |
10.65 |
348 |
0.404 |
13.70 |
|
Dichloro methane |
9.08 |
400 |
0.615 |
19.40 |
|
Bromo benzene |
5.4 |
347 |
0.417 |
13.81 |
|
Chloroform |
4.806 |
372 |
0.824 |
37.46 |
|
Benzene |
2.804 |
352 |
0.538 |
17.51 |
|
Toluene |
2.438 |
346 |
0.703 |
19.83 |
The results appear there is not any linear relation between dielectric constant of organic solvents and extraction efficiency but there is an effect for organic solvent structure on extraction efficiency which is surely demonstrated participation of organic solvent in the formation and stability of ion pair association complex12.
Interferences Effect
Extracted100μg Cd2+ from 5mL aqueous solutions at optimum conditions according to general method in the presence 0.01M interferences. The results were as in Table (3):
Table 3: Interferences effect
|
Foreign ion |
Abs. |
D |
|
Al3+ |
0.355 |
13.92 |
|
Ca+2 |
0.487 |
19.83 |
|
Mg+2 |
0.281 |
10.11 |
|
Cu+2 |
0.411 |
19.00 |
|
Hg+2 |
0.682 |
28.41 |
The results appear there is an interference effect for all metal cation used that is mean participation these metal cations to formation ion pair association complex which is consumption some of 8-HQ and cryptand C221 and to give arise to decline the concentration of 8-HQ than optimum necessary to formation ion pair complex with Cd2+ ion and giving decrease in extraction efficiency13.
Stoichiometry
By application two spectrophotometric method slope analysis and slope ratio according to general method the results demonstrated as in Figures 17, 18:
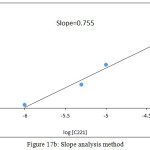 |
Figure 17b: Slope analysis method |
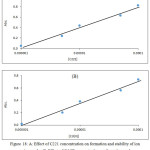 |
Figure 18: A: Effect of C221 concentration on formation and stability of ion pair complex. B: Effect of Cd (II) concentration on formation and stability of ion pair complex. |
From the slope ratio 1.825 and the slope in slope analysis 0.755 demonstrate the more probable structure of ion pair complex extracted was 1:1:1 [K-C221]+;Cd(OX)3–.
Spectrophotometric Determination
In spite of determination Cd2+ ion in different samples preparing calibration curve as in Figure 19:
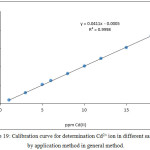 |
Figure 19: Calibration curve for determination Cd2+ ion in different samples by application method in general method. |
Conclusion
The optimum concentration of KOH base in aqueous solution giving higher extraction efficiency , its suitable for equilibrium formation anion complex of Cd(II), there is arising in ion pair association complex and extraction efficiency with increasing metal ion concentration, the optimum concentration of 8-HQ giving best equilibrium for formation ion pair association complex, there is an increase in extraction efficiency in the presence of methanol in aqueous solution, extraction method was endothermic, there is not any linear relation between dielectric constant of organic solvents and extraction efficiency but there is an effect for organic solvent structure on extraction efficiency, there is an interference effect for all metal cation used as interferences that is mean participation these cations to formation ion pair association complex which is consumption some of 8-HQ and cryptand C221, the more probable structure of ion pair complex extracted was 1:1:1 [K-C221]+;Cd(OX)3–.
References
- Asrafi, F.; Feyzbakhsh, A.; Heravi, N. E., J.O. International Journal of Chem. Tech. Research. 2009, 1(3), 420-425.
- Kudo, Y.; Katsuta, S.; Ohsawa, Y.; Nozaki, K., J.O. Thermodyn Catal. 2015, 6, 2, 1.
- Jawad, S.K.; Hameed, S.M., J.O. Ibn Al- Haitham J. for Pure & Appl. Sci.2011, 24 (2), 152-161.
- Jawad, S.K.; AL-Ghurabi, F., J.O. Babylon University/Pure and Applied Sciences. 2013, 21(2), 480-490.
- Jawad, S.K.; Muslim, N.M., J.O. Al-Qadisiya for Pure Science. 2014, 19 (2), 1-11.
- Jawad, S.K.; J.O. Thi -Qar University. 2010, 6(1), 7-22.
- Jawad, S.K.; Waday, F.Y.; Hameed, G.F., J.O. Kufa for Chemical Science. 2010, 1(1), 15-23.
- Marczenko, Z.; Balcerzak, M., Separation, preconcentration and spectrophotometry in inorganic analysis. 1st ed. Amesterdam: ELSEVIER, 2000.
- Jawad, S.K.; Azooz, E.A.; J.O. Research in Applied, Natural and Social Sciences. 2015, 1(2), 119-134.
- Atkins, P.; Paula, J. de. Physical Chemistry. 9th ed. Great Britain: Oxford University Press, 2010.
- Khammas, Z.A.A.; Ali, I.R; Jawad, S.K., J.O. Kufa for Chemical Science. 2012, 1(2), 67-85.
- Jawad, S.K.; MSC Thesis, Department of Chemistry, Education Collage Ibn Al-Haitham-Baghdad University, 1989.
- Jawad, S.K.; Abed, A.S., J.O. Natural Sciences Research. 2015, 5(7), 39-51.

This work is licensed under a Creative Commons Attribution 4.0 International License.









