Extraction Heavy Metals from Contaminated, Water using Chelating Agents
Khairia Mohamed Ahmed Al-Qahtani
Department of Chemistry, Princess Norah bint Abdel-Rahman University, Riyadh, Saudi Arabia.
Corresponding Author E-mail: dr_adalah@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330414
Water pollution caused bytoxicelements is one of the most important environmental problems in the world.Heavy metals are toxic to human and dangerous for the environment. The objectives of this study were: the effects ofchelating agentson heavy metals extraction from polluted water were carried out to examine.In different concentrations : 0.01, 0.05, 0.1 mg/l ofChelating agents: Ethylenediamine tetra acetate (EDTA), oxalic acid (OA) and citric acid (CA) were tested respectively .The effects of operating parameters, such as pH and extraction time were examined. The results showed that the extraction ability for mercury and copper from the polluted water decreased as follows: OA > CA > EDTA. Removal of metals was dependent on the concentration of extracting agent and raised with increasing concentration of chelating agents. the rate of metals removal were very rapid during first 50 min, and the removal decreased when the pH value of the solution increasing.
KEYWORDS:Heavy metal; chelating agent; EDTA; contaminated water
Download this article as:| Copy the following to cite this article: Al-Qahtani K. M. A. Extraction Heavy Metals from Contaminated, Water Using Chelating Agents. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Al-Qahtani K. M. A. Extraction Heavy Metals from Contaminated, Water Using Chelating Agents. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35265 |
Introduction
Pollution of water from industrial activities and domestic has not been regularly monitored and recorded as a problem. The development of industry and many activities such as mining, electroplating, refinishing, munitions , metalworking and manufacturing produce metal-contaminated by products that are responsible for a large amount of metal polluted sites in soils [1] .Soil and water pollution is becoming a significant industrial activities in the whole world[2] .Heavy metal pollutionhave been subjectedto both surface and subsurface environments [3].The most hazardous heavy metals:lead, Cadmium,copper, arsenic,mercury, zinc and chromium are contaminated soil and water[4] . Water maintains the life on the earth because it is the most vital liquid. About 97% water is not suitable for drinkingexists in oceans and about 3% is fresh water were 2.97% of it is glaciers and ice caps and the other 0.3% is available as a ground and a surface water for human use [5] .
Safe drinking water is a basic right of humans and a simple need for good health. In many part of the world fresh water resources is already limited. Because of the population increasing, urbanization and climate change, in the following century, fresh water will become even more limiting[6] . Because of thedischarge of untreated domestic and industrial wastewater into these resources, many cities in Asia facing increase in chemical material in drinking water[7]. Situation in South Asia is more aggravated, because of poor water quality and bad sanitation more than 0.5 million deaths of newborns happened per year with additional health threats. For example, in India (West Bengal) and some areas of Bangladesh, groundwater is polluted with arsenic (As) at levels as much as 70 times higher than the national drinking water quality standard of 0.05mg/l [8] . Worldwide, about 26% of all deaths are a result from infectious diseases caused by pathogenic bacteria and more people are dying from bad quality of water every year than from all types of physical violence including war[9,10].In India,lack of access to clean and safe water generated waterborne diseases unnecessarily by the residents of slums there[11] .Polluted water leads to death with waterborne diseases for infants and children such as diarrhea, while every fifth citizen suffers from illness and disease caused by this water [12] .
Toxic element pollution is a global problem, the common toxic element likePb, Cd, Hg, Cu and Coetc. are phytotoxic at both very high concentration as well as low concentration are detected in polluted water[13].These toxic elements reach the food chain through plants and aquatic animals when they presented in sediments and water[14]. In recent years there has been increasing need for estimating effect done by contamination of water. In this regard, there is growing interest in the use of Chelating agents like Ethylene diamine tetra acetate (EDTA), citric acid (CA) for detecting heavy metal ions, because of their sensitivity and selectivity [15] .The objective of the study was optimization of Experimental conditions for selected heavy metals (Cu and Hg) extraction from wastewater (polluted water) using aqueous solutions of oxalic acid , EDTA (ethylenediaminetetraacetic acid) and citric acid at different concentration and different pH.
Material and Methods
Chemical and Reagents
Al chemicals were pure (analytical grade) and were used without purification. oxalic acid (OA), ethylene di amine tetra acetic acid (EDTA) , citric acid,NaOH and HCl were obtained from BDH. The polluted water containing Cu and Hg ,Stock solution of Hg(ll) and Cu(II) (1,000 mg/l) were prepared to synthetic polluted water . All prepared solutionkept under acid conditions by adjusting the pH below2 using o.1 N HCl.The mixed heavy metals concentration was (10mg/l each). A total content of the analyzed heavy metals in contaminated water was determined and Content of heavy metals was detected by atomic absorption spectrometry (Model Z-8100 polarized Zeeman).
Extraction Procedure
The effect of removingheavy metals from water contaminated with mercury and copper (Prepared in the laboratory 10 mg /L of each) was studied. Four factors were identified for their effect on the extraction of heavy metals from contaminated water : 1) chelating agent; 2) reaction time; 3) chelating agent concentration; and 4) effect of pH on heavy metals extraction efficiencies. Three chelating agents (chelating ligands) were chosen: oxalic acid (OA) as a bidentate ligand : ligand withtwo donor atoms. citric acid(CA) as a tridentate ligand : ligand with three donor atoms.Ethylene di amine tetra acetic acid (EDTA) as a hexadentateligand : ligand with six donor atoms.
Batch extractions of heavy metals contaminants using a common extracting concentration of 0. 1M were conducted. The extraction experiments were placed in 25O mL flasks .The flasks containing 50 ml of polluted water by selected heavy metals and 50 ml of 0.1 M ( OA or CA or EDTA) were shaken in rotary shaker at agitation rate of 200 rpm and in an isothermal (250C) for 120 minutes.
The exclusionwere centrifuged at 3000 rpm for 10 min and the exclusion were then filtered through a Watman-42 filter paper for heavy metal analysis. Then the flasks returned to the shaker. Additionally, the effect of another concentration (0.05 M and 0.01) of chelating agents (EDTA, OA, CA) on the removal efficiency of selected heavy metals was investigated. Finally, the pH of the solutions after mixing was measured using a pH meter. The pH of solution was changed by adding 0.1N HCl or 0.1N NaOH solution in the rang (2-10).The pH of the solution was continuously measured and adjusted to 10 using 1.0N sodium hydroxide.The pH of the solution was adjusted to below 3 using 1.0N hydrochloric acid. The percent of metals removed was calculated using an equation similar to the one earlier reported [16] as:
Percent metal removed (%) = ( Ci – Co/ Co) × 100
where Co (mg /l) is the initial concentration, and Ci isthefinal concentration of element present in wastewaterafter extraction.
Results and Disscussen
Stability Metal Ion Complex Formation
Chelating agents were introduced into industrial applications such as ethylene diamine tetra acetic acid (EDTA) , citric acid (CA) and oxalic acid (OA). EDTA changes the metal ion removal ability.EDTA can form stable complexes with the transition elements due to EDTA was hexadentate ligand. In the reaction between metal ion and EDTA forms very stable complex. Because EDTA molecule contains of six donor atomsshield metal ion as a substitute for six water molecules. It means thatthe result was aqua complex to EDTA metal complex. The geometry of EDTA complex with metal becomes octahedral and the hybridization orbitals were (sp3d2) since six donor atoms. The bonding will be through nitrogen and oxygen (N. and O.) which is a donor atom with unshared electron paircan coordinate a metal ion. The value of pH increasesas each complexhydrogen of carboxylate ion (–COOH) or aqua ion (–OH2) is removed. EDTA reacts with metal ions, and forms a metal EDTA complex . The complex structures of EDTA and M (II) are shown in Fig. 1 [17].
In the same behavior the reaction between OA and CA with metal ions, donor atoms of OA and CA are oxygen (O) contain unshared electron pair, which can coordinate a metal ion. Citric acid consist of three donor atoms (O.) with unshared electron pair can coordinate a metal ion. Therefore, citric acid was considered to be tridentate ligand. Oxalic acid was classified as bidentate ligand because it contains tow donor atoms (O.).
Mn+ + EDTA → Metal – EDTA Complex
Mn+ + OA → Metal – OA Complex
Mn+ + CA → Metal – CA Complex
Research show high stability constants of some metal EDTA, CA and OA complexes .The values of stability constants are indicators of stability of metal complex; if the value was higher the stability of metal complex was high and it was difficult to break the metal bond with chelating agents and remove the metal [18].
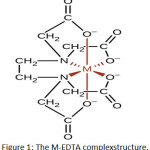 |
Figure 1: The M-EDTA complexstructure. |
Extraction Heavy Metals from Contaminated Water
Effect of Chelating Agent Concentration
The contaminated water by mercury and copper was prepared to see the ability of chelating agents to remove toxic heavy metals. EDTA is excellent chelating agent and low cost, so it is the most widely used in industrial applications. Large quantities of EDTA have been released into the environment, it is difficult to remove it.Removal of metals from environmental is a problem facing metal related industries.
The results of the 2-h batch removing of heavy metals from the contaminated water with EDTA, CA and OA are presented in Table.1.As concentration of EDTA increased, the percentage of Hg removal from polluted water increased reaching the highest value (60.3 , 63.2, 64.1 %) in ( 0.01, 0.05 ,0. 1 M) of EDTA. Relationship between percentage of Hg removed changes and EDTA concentration is almost linear. The behavior is similar in the case of citric acid(CA) and oxalic acid ( OA), as concentration of chelating agents increased, the percentage of Hg removed from polluted water increased reaching the highest value (74.3 , 75.1, 76.6 %) in ( 0.01, 0.05 ,0. 1 M) of OA and (69.9 , 71.1, 72.8 %) in ( 0.01, 0.05 ,0. 1 M) of CA . Relationship between percentage of Hg removal changes and chelating agents concentration is almost linear. Similarly to the behavior described for cupper are presented in Table. 1.
As concentration of chelating agents increased, the percentage of Cu removed from polluted water increased reaching the highest value (55.3 , 56.2, 59.5 %) in ( 0.01, 0.05 ,0. 1 M) of EDTA, (64.8 , 65.2, 66.6 %) in ( 0.01, 0.05 ,0. 1 M) of OA and (58.2 , 60.4, 62.8 %) in ( 0.01, 0.05 ,0. 1 M) of CA . Relationship between percentage of Hg removal changes and chelating agents concentration is almost linear.
Effect of Chelating Agent
The results of Experimental tests performed at different chelating agents concentrations show that oxalic acid and citric acid were more effective with respect to EDTA in removing mercury and copper from polluted water presented in Table. 1. The order of chelating agents in effective to remove heavy metals from contaminated water by mercury and copper were: OA >CA > EDTA. As concentration was (0.1M) and pH=4.00, the percentage of Hg removed from polluted water was 64.1% in the case of EDTA.While the percentage of Hg removed from polluted water was 72.8% in the case of CA and 76.6% in the case of OA. The reason for ordering the effectiveness of the chelating agents may be that it depends on the classification of the chelating agents as a ligands : OA (bidentate ligands) > CA (tridentate ligands) > EDTA(hexadentate ligands).
Studied metal removals from polluted water sample were in the order: Hg >Cu, as using EDTA,as concentration was (0.1M), the percentage of Hg removed from polluted water was 64.1% in the case of EDTA .While the percentage of Cu removed from polluted water was 59.9%.
Table 1: Values of percentage metals removal from polluted water by different chelating agents at pH=4.00 and room temperature.
|
Chelating Agents Co. |
Hg |
Cu |
||||
|
EDTA |
OA |
CA |
EDTA |
OA |
CA |
|
|
0.01M |
60.3 |
74.3 |
69.9 |
55.3 |
64.8 |
58.2 |
|
0.05M |
63.2 |
75.1 |
71.1 |
56.2 |
65.2 |
60.4 |
|
0.1M |
64.1 |
76.6 |
72.1 |
59.5 |
66.6 |
62.8 |
The Effect of Extraction Time
The effect of extraction time on selected heavy metals removal is shown in Fig.2. and Fig.3. indicates that the rate of metals removal were very rapid during first 50 min, and thereafter, the rate of metal removal remained stable. There were no significant increases found after 60 minutes of experiment and eventually it was the equilibrium time [19].
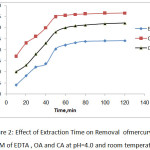 |
Figure 2: Effect of Extraction Time on Removal ofmercury by 0. 1M of EDTA , OA and CA at pH=4.0 and room temperature. |
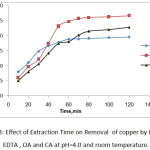 |
Figure 3: Effect of Extraction Time on Removal of copper by 0. 1M of EDTA, OA and CA at pH=4.0 and room temperature. |
Effect of pH
pH as a main factor has a dominant role in the removal of heavy metals. The removal of Hg and Cu from polluted water decreased with the rising pH value of the chelating agents solution. When the pH values were (2- 4), the removal of selected heavy metals were high .When the pH values were above 4, the removing heavy metals decreases as solution pH increased.Inmany previous studies have been shown that the pH value is an important factor controlling the removal of heavy metals from contaminated solutions [20].
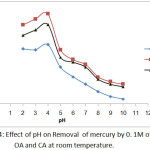 |
Figure 4: Effect of pH on Removal of mercury by 0. 1M of EDTA, OA and CA at room temperature. |
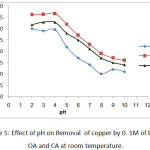 |
Figure 5: Effect of pH on Removal of copper by 0. 1M of EDTA , OA and CA at room temperature. |
The contents of Cu and Hg extracted decreased Whenthe pH value of chelating agents solution increased from 5 to 10. Generally, when pH value of the solutionincreasesthe removal of heavy metals will decrease, as many metal complex has lower value of solubility at high value of pH.when pH value of EDTA, OA and CA solution increased,the extraction amount of the two metals decreased.
Coucluion
The studied contaminated water by high concentration of mercury and cupper. Both analyzed chelators: EDTA, oxalic acid and citric acid were efficient for selected heavy metals removal from polluted water . Removal of analyzed metals was dependent on the concentration of extracting agent and raised with increasing concentration of chelators. The results of experimental tests tested at different chelators concentrations show that oxalic acid was more effective in removing selected heavy metals thancitric acid and EDTA.The removal of the tested metals depended on the pH value of the solution. The removal of heavy metals from polluted water decreased with the increasing pH value of the chelating agents solution.
References
- Tejowulan, R.S. ;Hendershot, W.H. Removal of trace metals from contaminated soils using EDTA incorporating resin trapping techniques, Environmental Pollution.1998, 103(1), 135-142.
CrossRef - Pociecha, M. ;Lestan, D. Using electrocoagulation for metal and chelant separation from washing solution after EDTA leaching of Pb, Zn and Cd contaminated soil, Journal of Hazardous Materials. 2010, 174(1-3), 670-678.
CrossRef - Peters, R.W. Chelant extraction of heavy metals from contaminated soils, Journal of Hazardous Materials. 1999, 66(1-2), 151-210.
CrossRef - Abumaizar, R.J. ; Smith E.H. Heavy metal contaminants removal by soil washing, Journal of Hazardous Materials.1999, 70(1-2), 71-86.
CrossRef - Miller, G. T. Jr. Environmental Science: Working with the Earth. (6th Ed.). California: Wadsworth Publishing Company, (Chapter 11),1997.
- Jackson, R. B. et al. Water in Changing World. Issues in Ecology, 9, Washington, DC: Ecological Society of America. 2001, 1-16.
- Annachhatre, A. P. Water Quality and Wastewater Management. In J. K. Routray and A. Mohanty (Eds.), Environmental Management Tools: A Training Manual, pp. 125-129, United Nations Environment Programme (UNEP) & Asian Institute of Technology (AIT), Thailand: School of Environment, Resources and Development.2006.
- UNEP. Global Environment Outlook 2000. New York and London: United Nations Environment Programme (UNEP),1999.
- WHO. Global Strategy for Food Safety: Safer Food for better Health. Geneva: World Health Organization (WHO),2002.
- UNEP GEMS/Water Programme.Water Quality for Ecosystem and Human Health.(2nd Ed.). Ontario: United Nations Environment Programme Global Environment Monitoring System (UNEP GEMS)/Water Programme.2008.
- Lal, P. et al. Incidence of Diarrhea and some related Environmental and Behavioural Factors in Jhuggis of Delhi. Indian Journal of Public Health.1996,40(2).
- Kahlown, M. A.; Tahir, M. A.; Rasheed, H.;Bhatti, K. P. Water Quality Status, National Water Quality Monitoring Programme. 4th Technical Report. Pakistan Council of Research in water Resources (PCRWR). 2006,5.
- Divya, S.;Archana, T. ;Richa G. Phytoremediation of lead from wastewaterusing aquatic plants”Journal of Agricultural Technology.2012, 8(1): 1-11.
- Veronica, B.; Grazia, M.; David G. ; Brunello, C.; Renato, I.Enhanced Heavy Metal Phytoextraction from Marine Dredged Sediments ComparingConventional Chelating Agents (Citric Acid and EDTA) with HumicSubstances. Journal of Water Air Soil Pollut Springer.Science.2008.
- Amin, M. Phytoremediation of heavy metals from municipal wastewater byTyphadomingensis. African Journal of Microbiology Research.2012, 6(3), 643-647.
- El-Ashtoukhy, E.S.; Amin, N.K. ;Abdelwahab, O. Removal of lead(II) and copper(II) from aqueous solution using pomegranate peel as a new adsorbent, Desalination. 2008,223: 162-173.
CrossRef - Dupare,D. B. Detection of Heavy Metal Ions from Water using Conventional Chelating Agents (Citric Acid And EDTA) in and around Murtizapur Region. International Journal of Chemical and Physical Sciences.2015,4:30-37.
- Sultan, I.; Amer. M. Simplified Removal of Chelated Metals. AQUACHEM INC. 2011, 1102(4):1-5.
- Karthikeyan, S.; Balasubramanian, R.;Iyer, C.S.P. Evaluation of the marine algae Ulvafasciata and Sargassum sp. for the biosorption of Cu(II) from aqueous solutions. Bioresource Technology. 2007,98(2), 452-455.
CrossRef - Yang, J. ;Klarup, D. Extraction Kinetics of Copper, Zinc, Iron, and Manganese from Contaminated Sediment Using Disodium Ethylenediaminetraacetate.Water Air and Soil Pollution. 1994, 75: 205-225.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









