Co2 Adsorption Study on Nio and Pr2o3 -Nio Catalyst Synthesis Using Simple Sol-Gel Method
Mohd Hasmizam Razali1, Uwaisulqarni Ismail1, Mohd Zul Helmi Mohd Rozaini2 and Mahani Yusoff3
1School of Fundamental Sciences, Universiti Malaysia Terengganu,21030 Kuala Terengganu, Terengganu, Malaysia.
2Institute of Biotechnology Marine, Universiti Malaysia Terengganu,21030 Kuala Terengganu, Terengganu, Malaysia.
3Faculty of Earth Science, Universiti Malaysia Kelantan KampusJeli,KarungBerkunci No.100, 17600 Jeli,Kelantan, Malaysia.
Corresponding Author E-mail: mdhasmizam@umt.edu.my
DOI : http://dx.doi.org/10.13005/ojc/330431
Nickel oxide (NiO)and praseodymium oxide mixed nickel oxide (Pr2O3-NiO) catalysts was synthesized using modified sol-gel method for CO2 adsorption. The synthesized catalysts was characterized using x-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive of x-ray (EDX) and N2 gas adsorption-desorption to study their physical and chemical properties. The capability of catalystto adsorb CO2 was tested using temperature programmed desorption of CO2 (TPD-CO2) and their interaction was also studied.It was found that, Pr2O3-NiO catalyst have a remarkable CO2 uptake capacity (331.40 µmol/g) as compared to NiO (32.53 µmol/g). This is governed by the presence of cubic Pr2O3 and less crystalline sample. Moreover smaller particle size and larger BET surface area of Pr2O3-NiO catalyst provided good chemical interactions between Pr2O3-NiO and CO2 molecules.
KEYWORDS:Carbon dioxide; adsorption; nickel oxide; praseodymium oxide; catalyst; sol-gel
Download this article as:| Copy the following to cite this article: Razali M. H, Ismail U, Rozaini M. Z. H. M, Yusoff M. Co2 Adsorption Study on Nio and Pr2o3 -Nio Catalyst Synthesis Using Simple Sol-Gel Method. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Razali M. H, Ismail U, Rozaini M. Z. H. M, Yusoff M. Co2 Adsorption Study on Nio and Pr2o3 -Nio Catalyst Synthesis Using Simple Sol-Gel Method. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35172 |
Introduction
Over the past few years, the amount of carbon dioxide (CO2) in the atmosphere are exponentially increasing and it is predicted to follow a similar trend in the future.Atmospheric carbon dioxide (CO2) levels have grown exponentially in the last two centuries as a consequence of the larger anthropogenic CO2 emissions due to the high demand of fossil fuels by an increment of the world population and the industrial development. This fact has caused that levels of atmospheric CO2 increase to above 400 ppm, which has entailed global warming and ocean acidification [1]. This increment is significant and it was expected to increase further due toenergy demand for economic and population growth. As known, at this moment the fossil fuels are the dominant energy resources which provide 86% share in the global energy utilization [2]. This large utilization of fossil fuels accounts for 75% carbon dioxide (CO2) emissions to the atmosphere from various industries such as fossil fuelled power plants, cement industry, refinery and synthetic ammonia production units [3]. Burning of fossil fuels for transportation, electricity and heat are responsible for almost all of the increase of CO2 in the atmosphere over the last 100 years.
As CO2 is the main greenhouse gas (GHG) and the root cause of global warming which influence the climate change [4], extensive studies into creating effective CO2 capture solution to mitigate CO2 emission is explored.Among the known methods, adsorption shows the best results in terms of efficiency, energy costs and versatility to different compounds [5]. Thus, the development of novel adsorbent materials for CO2 adsorption is a greatly concerned step for practical CO2 capture and storage (CCS)applications. In general, ideal adsorbents should have a high CO2 adsorption capacity, excellent adsorption selectivity over other gases, and a good chemical and mechanical stability. Recently, wide variety of porous materials including mesoporous silica [6], amine functionalized mesoporous silica [7], mesoporous alumina [8], metal organic frameworks (MOFs) [9-11], activated carbon [12] as well as mesoporous carbons [13]have been tried as solid adsorbents for CO2. However the performance of these materials for CO2 adsorption is still low. Latest study by Li et al., (2017), shows that the modification of mesoporous carbon by nickel oxide had increased the adsorption capacityof CO2 and their selectivity due to better interaction between CO2with the introduced metal oxides [14]. This finding suggested that metal oxide has a great potential to be used as CO2adsorbent. Thus in this study nickel oxide (NiO) and praseodymium oxide mixed nickel oxide (Pr2O3-NiO) has been synthesized and applied for CO2 adsorption. The interaction of NiO and Pr2O3 with CO2 were also investigated.
Experimental
Catalyst Preparation
Pr2O3-NiO was prepared using simple sol-gel method. 2.00 gof Ni(NO3)2•6H2Owas dissolved with minimum amount of distilled water and stirred for 15 minutes. Then, 2.00 g of Pr(NO3)3•6H2O was added and continuously stirred. The mixture was then transferred into an evaporating disc and aged in an oven at 80oC for 24 hours. A greenish solid gel was formed and calcined at 400°C for 2 hours.NiO catalyst was prepared using similar procedure without the addition of Pr(NO3)3•6H2O.
Catalyst Characterization
The crystal structure was studied using X-Ray diffraction (XRD), Bruker D8 Diffractometer with Cu-Kα (λ = 1.54021 Å) and scans were performed in step of 0.2o/second over the range of 2θ from 10 up to 70o.The morphology of samples was observed via SEMusing Philip XL 40.N2gas adsorption isotherm was taken after pre-treatment in vacum at 473 K and surface area was analyzed from the isotherm using Brunauer-Emett-Teller equation.
Catalytic Performance
Temperature Programmed Desorption of CO2 (TPD-CO2) was recorder on a Thermo-Finnigan TPD/R/O 1100 fitted with a thermo-conductivity detector (TCD) and controlled by a computer. In order to remove surface contaminants, the sample (0.1 g) loaded in a quartz reactor was pre-treated at 100oC in a nitrogen stream for 1 hour. After cooling to room temperature, a flow of 5% ml/min (30ml/min) of CO2 gas was passed through the sample and the temperature was raise at the rate of 10oC/min form room temperature up to 600oC, while the TCD signal was recorded.
Results and Discussion
Figure 1 shows the XRD pattern of NiO and Pr2O3-NiO catalyst. For NiO catalyst, three sharp and narrow peaks were observed at 2θ of 37.24, 43.27 and 62.87o with d values of 2.41, 2.09 and 1.48 Å (Figure 1a). These peaks can be indexed as (1 1 1), (2 0 0), and (2 2 0)crystal planes of the crystalline cubic NiO[15]. Sharp and narrow diffractionpeaks indicating that NiO catalyst sample is highly crystalline because the sample was calcined at high temperature (400°C). During the calcination, the crystallite size NiOincreased, thus enhanced the crystallite growth in order to minimize the interfacial surface energy[16].On the other hand, broad and big XRD peaks was observed for Pr2O3-NiO catalyst (Figure 1b). Besides the peaks matched to cubic NiO phase at 2θ of 37.46, 43.78, 63.50 and one additional peak was appeared at 2θ of 28.54° with d values of 3.14 Å which is corresponded to cubic Pr2O3 (PDF 1995 d values (Å): 3.13 Å) [17]. Broad and big peaks suggesting that Pr2O3-NiO sample is less crystalline as compared to pure NiO. This is probably due to the present of Pr2O3, which can prevent the agglomeration of particles by occupying the position of nickel oxide [18]. The XRD pattern also revealed the absence of binary or ternary compounds as NiO and Pr2O3existed as individual structure proved that solid state reaction did not occur.
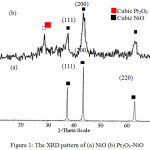 |
Figure 1: The XRD pattern of (a) NiO (b) Pr2O3-NiO |
Figure 2 shows the SEM micrograph of NiOand Pr2O3-NiO catalysts. As shown figure 2(a), NiO catalyst particlestend to accumulate, which resulted in the smooth catalyst surface and pack particles. Upon the addition of Pr2O3, an irregularity of the particle shape was observed in which smaller particles were dispersed within the matrix of larger particles (Figure 2b). The near spherical shape particles were uniformly distributed on the catalyst surface. The surface of resultant sample is relatively rough without obvious agglomeration phenomenon. This indicated that the SEM results of Pr2O3-NiO catalyst is in good agreement with the XRD analysis which exhibited very broad peaks denoting a less crystalline character as compared to NiO catalyst.
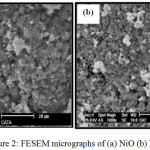 |
Figure 2: FESEM micrographs of (a) NiO (b) Pr2O3-NiO |
Figure 3(a) illustrated the EDX spectra of NiO catalyst, which revealed that the sample is only consist of Ni and O elements existed as NiO compound which is consistent with XRD analysis. Nickel and oxygen emitted the X-ray signal at 7.386 kV and 0.886 kV respectively. Meanwhile, the EDX spectra of Pr2O3-NiO demonstrates the presence of Pr, Ni and O elements without other impurity elements (Figure3(a)). Praseodymium emitted the X-ray signal at 5.166 kV.
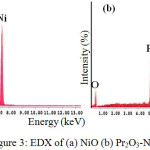 |
Figure 3: EDX of (a) NiO (b) Pr2O3-NiO
|
The N2 adsorption-desorption isotherms of catalysts are illustrated in Figure4. In this research, all studied catalysts exhibited typical type IV isotherms with hysteresis loops attributing to capillary condensation in mesopores, which further demonstrates that the catalysts have mesoporous structure. It is noteworthy that the types of hysteresis loop between NiO and Pr2O3-NiO catalysts are slightly different. The NiOshowed H1-type hysteresis loop, while Pr2O3-NiO catalysts exhibited H2-type hysteresis loop. Type IV isotherm with H2-type hysteresis loop is closely associated with the “ink-bottle” pore structure, which leads to a not well-defined pore shape. Meanwhile, type IV isotherm with H1-type hysteresis loop appears in mesoporous materials comprising nearly spherical-shaped particles [19]. The BET surface area of NiO and Pr2O3-NiO catalysts was found to be 5.60 m2/g and 75.58 m2/g, respectively. Larger BET surface area of Pr2O3-NiO attributed to their smaller particle size, as Pr2O3suppressed the grain growth of NiO.
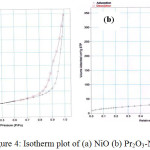 |
Figure 4: Isotherm plot of (a) NiO (b) Pr2O3-NiO |
Figure 5 shows the TPD profile of CO2 desorption on NiO catalyst. Three desorption peaks at 90oC, 140oC and 240oC was observed. These three peaks were attributed to the existence of three desorption sites, corresponding to different interaction of CO2 with NiO. First peak at 90°C was assigned to two dative covalent bonds between oxygen from CO2 with Ni2+. Each oxygen atom donated lone pair electrons and occupied hybrid orbital of Ni2+ to form pure oxygen coordination as shown in figure 6[20]. As a results constrained coordination sphere was formed due to weak dative covalent bonds, therefore CO2gas can be easily desorbed and released at low temperature.
 |
Figure 5: TPD profile of CO2 desorption onNiO catalyst. |
![Figure 6: Coordination of CO2 to Ni2+ via pure oxygen coordination [19].](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Ads_Moh_fig6-150x150.jpg) |
Figure 6: Coordination of CO2 to Ni2+ via pure oxygen coordination [19]. |
The second peak at 170oC was attributed to the pure covalent bond between carbon from CO2with Ni2+ in NiO (figure 7) [19]. This bonding was formed when single electron of CO2combined with the unpaired d electron on the Ni atom in its d configuration. This bonding is strong because the existence of pure covalent bond thus CO2desorb at higher temperature.
![Figure 7:Coordination of CO2 to Ni2+ via pure carbon coordination [19].](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Ads_Moh_fig7-150x150.jpg) |
Figure 7:Coordination of CO2 to Ni2+ via pure carbon coordination [19]. Click here to View figure |
The third peak was appeared at the highest temperature of 240oC owing to the mixed carbon-oxygen coordination (figure 8) [19]. The highest desorption temperature of this coordination maybe due to the existence of both pure covalent bond and dative covalent bond. The pure covalent bond was formed from the sharing of electrons between the Ni and electron from the carbon. In addition, the lone pairs from the oxygen were donated into the diffused sp hybrid orbitals of Ni to form dative covalent bond.
![Figure 8: Coordination of CO2 to Ni2+ via mixed carbon-oxygen coordination [19].](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Ads_Moh_fig8-150x150.jpg) |
Figure 8: Coordination of CO2 to Ni2+ via mixed carbon-oxygen coordination [19]. |
The TPD profile of CO2 desorption obtained for Pr2O3-NiO catalyst asshown in figure 9. It is slightly different with NiO catalyst, as four peaks was observed at temperature of 100°C, 190°C, 240°C and 440°C.
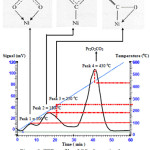 |
Figure 9: TPD profile of CO2 desorption by NiO-Pr2O3 catalyst. Click here to View figure |
The first three peaks at lowertemperature corresponded to the three peaks as observed in NiO catalyst. That peaks related to the chemical interaction between NiO and CO2. An additional peak at 440°C is belong to Pr2O3 desorption sites, suggesting that incorporation of Pr2O3 species in NiO increased the adsorption site of CO2.In fact, CO2 adsorption site on Pr2O3 is more than NiO, as the signal of forth peak is the highest. The presence of Pr2O3 created a tight interface interaction between NiO-Pr2O3catalyst and CO2. This is due to the weak basicity of Pr2O3 which resultedin the formation of carbonates. In fact, this is a common reaction in the acid base interaction to give an unstable surface carbonate species as an intermediate (Equation 1).
Pr2O3 + CO2 → Pr2O2CO3 (Equation 1)
As a results, the amount of CO2adsorbed on Pr2O3-NiO catalyst was found to be 10 times greater than NiO catalyst as shown Table 1.
Table 1: The amount of adsorbed gas by the catalysts.
|
Catalyst |
Amount CO2 gas adsorbed (μmol/g) |
|
NiO |
32.53 |
|
Pr2O3-NiO |
331.40 |
Conclusion
CO2 adsorption studies over NiO and Pr2O3-NiO catalysts were carried out. Both catalysts was synthesized using simple sol-gel method. Pr2O3-NiO catalyst possessed less crystallinity as compared to NiO catalyst. Somehow, the CO2 adsorption performance was found better for Pr2O3-NiO than NiO. The introduction of Pr2O3 increased the adsorption capacity of the samples because more CO2 adsorption sites presence on the catalyst. Moreover, large surface area of Pr2O3-NiO support their good performance for CO2 adsorption.
Acknowledgement
The authors are grateful to Universiti Malaysia Terengganu (UMT) for providing the facilities to carry out this project and Ministry of Higher Education of Malaysia for the financial support vote FRGS 59358.
References
- Yu, C.H.,Huang, C.H.,Tang, C.S.,Aerosol Air Qual. Res.2012, 12,745-769.
CrossRef - Bhagiyalakshmi, M., Hemalatha, P., Ganesh, M., Mei, P., Jang, H.T., Fuel,2011, 90, 1662–1667.
CrossRef - Abdul Rahman, F., A. Aziz, M., M., Saidur, R., Wan Abu Bakar, W. A., Hainin, M.R., Putrajaya, R., Abdul Hassan,N.,Renewable and Sustainable Energy,2017,71, 12-126
- Lastoskie C., Science,2010, 330,595–601.
CrossRef - Glover,T.G.,Dunne,K.I.,Davis,R.J.,LeVan,M.D., Micropor. Mesopor. Mater.2008,111, 1-11.
CrossRef - Ahmed, S., Ramli, A., Yusup, S., Farooq, M., Chemical Engineering Research and Design, 2017,122, .33-42
- Wang, F., Gunathilake, C., Jaroniec, M. Journal of CO2Utilization, 2016, 13,114-118.
- Sumida, K.,Rogow, D.L.,Mason, J.A.,McDonald, T.M.,Bloch, E.D.,Herm, Z.R.,Bae, T.,Long, J.R., Chem. Rev. 2012,112, 724-781.
CrossRef - Sargazi, G.,Afzali, D., Mostafavi, A., Ebrahimipour. S. Y., Journal of Solid State Chemistry, 2017,250, 32-48.
CrossRef - Rezaei, F., Lawson, S., Hosseini, H., Thakkar, H., Hajari, A., Monjezi, S., Rownaghi, A. A., Chemical Engineering Journal, 2017,313,1346-1353.
CrossRef - Ammendola,P.,Raganati,F.,Chirone, R., Chemical Engineering Journal, 2017,322, 302-313.
CrossRef - Huang, K., Chai, S.H., Mayes, R.T., Tan, S., Jones, C.W., Dai, S., Microporous andMesoporousMaterials, 2016,230, 100-108.
- Li, M., Huang, K., Schott, J.A., Wu, Z., Dai, S., Microporous and Mesoporous Materials, 2017,249, 34-41.
CrossRef - Dharmaraj,N.,Prabu,P.,Nagarajan,S.,Kim, C.H.,Park,J.H.,Kim,H.Y., Mat. Sci. Eng. B,2006, 128, 111–114.
CrossRef - Kingery,W.D.,Bowen, H.K.,UhlmannD.R., Introduction to Ceramics. John Wiley and Sons, New York, 1976,522
- Razali, M.H., Physical and catalytic study of nickel oxide based catalyst for methanation reaction of natural gas. M Sc. Thesis, UniversitiTeknologi Malaysia, 2005, 47-51
- Razali, M.H., Wan Abu Bakar, W.A., Buang, N.A., Journal of Sustainability Science and Management,2010., 5,, 148-152.
- Sing, K.S.W.,Everett,D.H.,Haul,R.A.W.,Moscou,L.,Pierotti, R.A.,Rouquerol, J.,Siemieniewska, T.,Pure Appl. Chem.1985,57,603–619.
CrossRef - Williams M. A., Catalytic Activation of Carbon Dioxide. Washington D. C.: American Chemical Society.1988,104-118.

This work is licensed under a Creative Commons Attribution 4.0 International License.









